Cas9 Nuclease
NLS-SpCas9-NLS Nuclease Solution
Akron’s recombinant NLS-SpCas9-NLS (Cas9) Nuclease Solution is manufactured following all relevant cGMP guidelines for ancillary materials. It is purified using pharmaceutical techniques without the use of histidine tags and nickel columns. Akron’s Cas9 Nuclease is a single-chain, 162 kDa, non-glycosylated DNA endonuclease expressed in E. coli. The amino acid sequence comes from Streptococcus pyogenes, and the protein structure has been altered by the addition of a nuclear localization sequence (NLS) to both the N- and C- terminals of the protein, ensuring it is effectively imported into the cell nucleus.
Active Substance
• NLS sequences added to both the N- and C- terminal
• Effective site-directed nuclease activity
• Carrier protein-free formulation
Manufacturing
• Tag-free pharmaceutical processing
• Sterile filtration and aseptic filling
• Animal free expression system with E. coli host
Quality
• Relevant cGMP guidelines used in manufacture, testing, and release
• USP <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
• EP 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products
• ISO 20399:2022, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products
Stability
• Store at -80 °C
• Keep out of light and avoid repeated freeze-thaw cycles
• Ships with Dry Ice
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
DISCLAIMER: Currently available as Catalog # PD1019, for research use only, not for further manufacturing.
- Appearance (Visual Inspection)
- Protein Concentration (UV Spectrophotometry)
- Purity (RP-HPLC)
- Impurities of Upper Molecular Weight (SDS-PAGE)
- Impurities of Lower Molecular Weight (SS-PAGE)
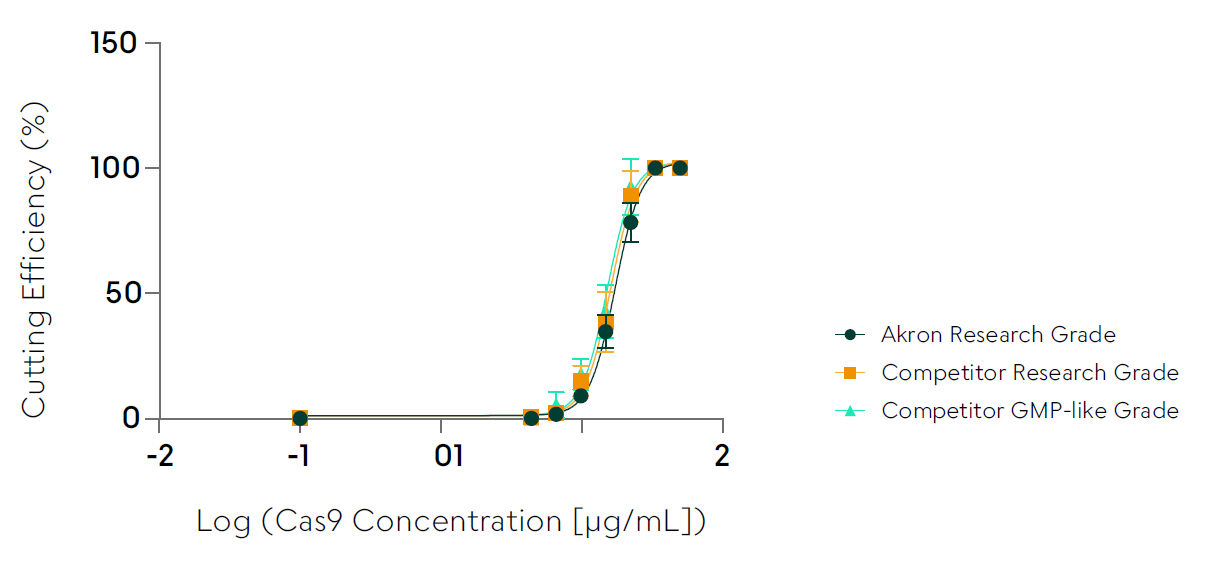
- Activity, in vitro (Percent Cutting Efficiency by SEC UPLC)
1. Under what conditions should Akron’s Cas9 Nuclease Solution be stored?
Cas9 Nuclease should be stored at -80 ºC.
2. Can the Akron Cas9 Nuclease undergo multiple freeze-thaw cycles?
Please avoid multiple freeze-thaw cycles.
Aliquot Sizes & Formats
RUO Solution (1 mg) Cat. # PD1019