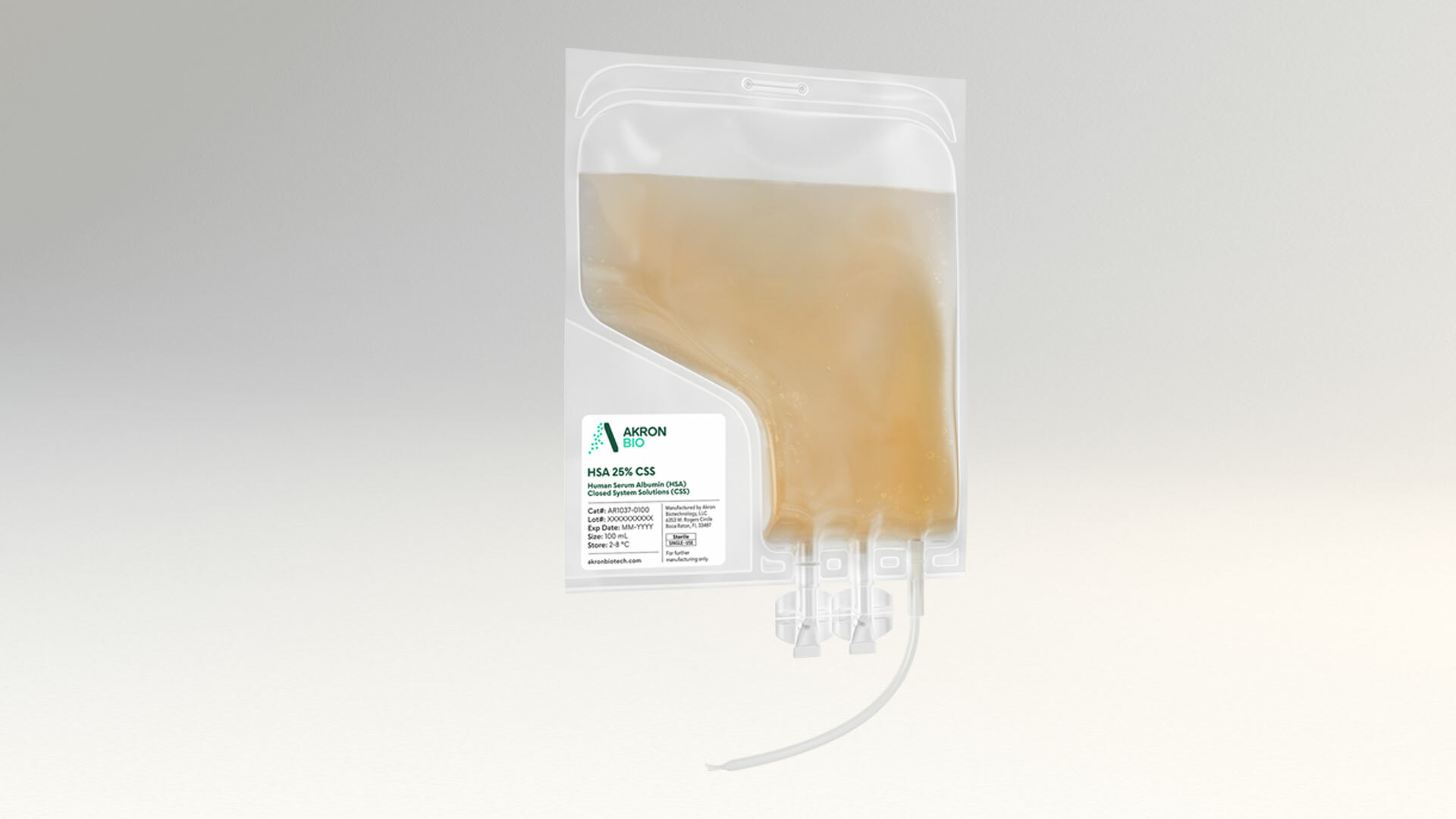
cGMP HSA 25% Solution
Human-plasma derived cGMP albumin optimized for cell therapy manufacturing.
Akron’s Human Serum Albumin (HSA) 25% Solution is manufactured, tested, and released following relevant cGMP guidelines for blood-derived ancillary materials and supported by a Type II Master File (MF) on file with the FDA which can be referenced during your drug or biologic application process. Akron’s HSA 25% Solution is specifically formulated for cell and gene therapy commercial manufacturing.
Akron’s HSA 25% Solution uses raw material sourced from US donors, collected in FDA-licensed facilities adhering to all donor screening and virus testing legislation set forth in US 21 CFR 610.40. Redundant pathogen testing occurs during the manufacturing process to ensure safety. Sterile filtration and aseptic filling with Endotoxin and Sterility testing performed per USP/EP and Mycoplasma testing performed per EP on the final product. Akron’s HSA 25% Solution does not contain stabilizers typically found in other pharmaceutical albumin solutions because these substances (caprylate & acetyltryptophanate) have been shown to interfere with relevant cell culture. Their exclusion allows for an HSA supplement that promotes optimal cell culture performance for the human cell therapy industry.
Raw Material
- Human Plasma from US-licensed plasma donation centers
- Donor screening and virus testing per 21 CFR 610.40
- cGMP Raw Material – Cohn Fraction V
Manufacturing
- Type II eCTD DMF (#019354) on file with FDA
- Formulated with WFI (water for injection)
- Does not contain stabilizers Caprylate or N-Acetyl-L-Tryptophanate
- No animal-derived materials are used in the manufacture of this product
- Sterile microfiltration followed by aseptic filling
Quality
- Relevant cGMP guidelines used in manufacture, testing, and release
- USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
- ISO 20399:2022, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products
- High Purity, Low Endotoxin - Endotoxin, and Sterility testing per USP/EP; Mycoplasma testing per EP
Stability
- 24-month shelf life
- Store at 2-8 °C
- Transport with cold packs
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
• Appearance
• pH
• Osmolality
• Concentration
• Purity
• Mycoplasma
• Bacterial Endotoxins
• Sterility
1. Why use Human Serum Albumin (HSA) 25% Solution?
Akron’s Human Serum Albumin (HSA) 25% Solution is manufactured, tested, and released following relevant cGMP guidelines for blood-derived ancillary materials and supported by a Type II Master File (MF) with the FDA. Additive and typical stabilizers, which have been shown to interfere with cell culture, are not included in Akron’s HSA 25% Solution, making this product ideal for human cell therapy commercial manufacturing. Redundant pathogen testing occurs during the manufacturing process to ensure safety. Sterile filtration and aseptic filling are performed with Endotoxin and Sterility testing performed per USP/EP and Mycoplasma testing performed per EP.
2. What are the recommended storage conditions for HSA 25% Solution?
We recommend storing these products at 2-8 °C.
3. What are the shipping conditions for HSA 25% Solution?
These products ship with cold packs.
4. What is the shelf-life for HSA 25% Solution?
This product meets the industry standard of a 2-year shelf-life.
5. Where is the raw material sourced?
All human plasma units used as raw material are sourced from FDA-licensed collection facilities with donors traceable to the U.S.
6. How are the raw material donations screened?
Individual donor identification, registration, and education takes place, per 21 CFR 630.10. Each plasma unit used as raw material is virus tested, per 21 CFR 610.40, at the time of collection and found negative or non-reactive for Hepatitis B surface Antigen (HBsAg), antibodies against Human Immunodeficiency Virus (HIV)- 1 and 2 (anti-HIV 1/2), and antibodies to Hepatitis C Virus (anti-HCV). Each donor unit also undergoes serological testing for antibodies against Treponema pallidum (Syphilis), and Nucleic Acid Testing (NAT) for Hepatitis A Virus (HAV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus 1 (HIV-1), and Parvovirus B19 (B19V).
7. Do you have a Master File (MF) available for this product?
Yes, our HSA 25% Solution has a Type II eCTD MF (#019354) on file with the FDA. Upon request, we can provide you a Letter of Authorization, which permits the FDA to cross-reference our MF in support of your regulatory submissions.
8. Do you have a Safety Data Sheet (SDS) available for this product?
Yes, an SDS is available upon request.
9. Is this an injectable-grade or clinical-grade material?
No, this product is not for direct use in humans. See intended use below.
10. What is the intended use for the product?
For research use or further manufacturing use in ex vivo cell therapy applications only. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
11. Can this product be used to manufacture a vaccine?
It is up to the drug product manufacturer to determine if this material fits their required specifications for further manufacturing use in their drug manufacturing process. As stated above, this product is not sold to be injected directly into humans, even though Akron’s HSA 25% Solution is manufactured using WFI (water for injection) and has a Type II MF on file with the FDA (CBER) you can reference in your filings.
12. Are stabilizers present in this HSA solution?
No, our HSA 25% Solution does not contain the stabilizers, sodium caprylate or sodium acetyltryptophanate, typically found in other pharmaceutical albumin solutions. Our formulation is intended for optimal cell culture performance and is not being sold for direct injection into humans.
13. How does the absence of stabilizers in Akron’s HSA 25% Solution affect performance relative to clinical albumin products?
The major stabilizers found in clinical albumin preparations, per the Human Albumin USP monograph, are sodium acetyltryptophanate and sodium caprylate (also known as sodium octanoate). These stabilizers bind to the albumin molecule and help to protect it against oxidation and heat damage, especially during the typical pasteurization step included in clinical human albumin preparations. Decomposition and metabolic products of acetyltryptophanate have shown to reduce cell performance when present in culture and to induce apoptosis of certain immune cells.2, 3 Caprylate has also been shown to slow growth and lower colony formation when present in stem cell culture. Our Human Serum Albumin 25% Solution is manufactured without these stabilizers and without any other additives, giving your cells the best chance of a successful culture.
14. Is this product compendial grade / USP-grade?
Akron’s cGMP AK8228 HSA 25% Solution is tested and released upon meeting specifications in line with EP, with Endotoxin & Sterility testing per USP/EP and Mycoplasma testing per EP. However, the product itself is not compendial grade material. The HSA monographs are written with the perspective of an injectable material, but we focus on providing material optimized for cell and gene therapy manufacturing. Our HSA 25% solution is not for direct use in humans and is not fit for intravenous use.
15. What packaging options are available?
This product comes packaged in Type I borosilicate glass vials closed with a rubber stopper and sealed with an aluminum flip-off cap seal. The vials are designed for single use, as once the seal and stopper are removed, the vial contents are no longer considered sterile. We also offer our HSA solution in sterile single-use bags designed for closed-system commercial cell culture systems (see product AR1037). Packaging for this product can be customized, with different packaging materials and/or fill volumes
available under contract.
16. Do I need to use a sterile needle and syringe to remove the product from the vial?
No, our vial packaging allows easy access with a laboratory pipette.
Albumin is the most abundant protein in blood plasma and has historically been used in cell culture for its ability to support mammalian cell growth. Albumin is known to carry many important substances including lipids, amino acids, hormones, peptides, metals, and other undefined low molecular weight molecules. It also offers antioxidant protection to cells and participates in the transport and signal mechanics of hormones and growth factors. Akron’s cGMP-compliant HSA 25% Solution can be used in a wide range of applications such as cell culture supplementation, assay standardization, protein stabilization, and product formulation.
Aliquot Sizes & Formats
Vials (100 mL) Cat. # AK8228-0100
Liquid CSS (100 mL) Cat. # AR1037-0100