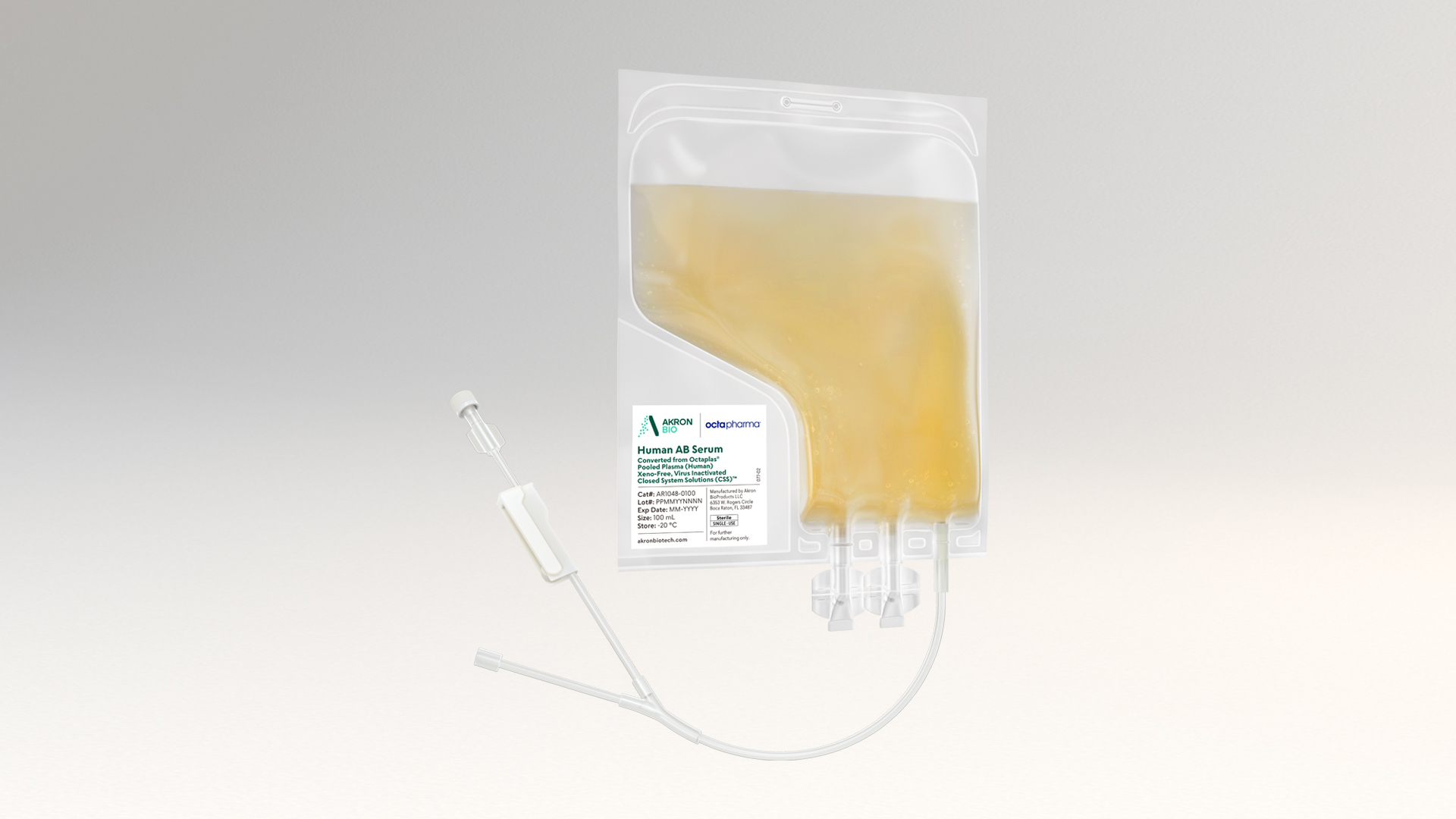
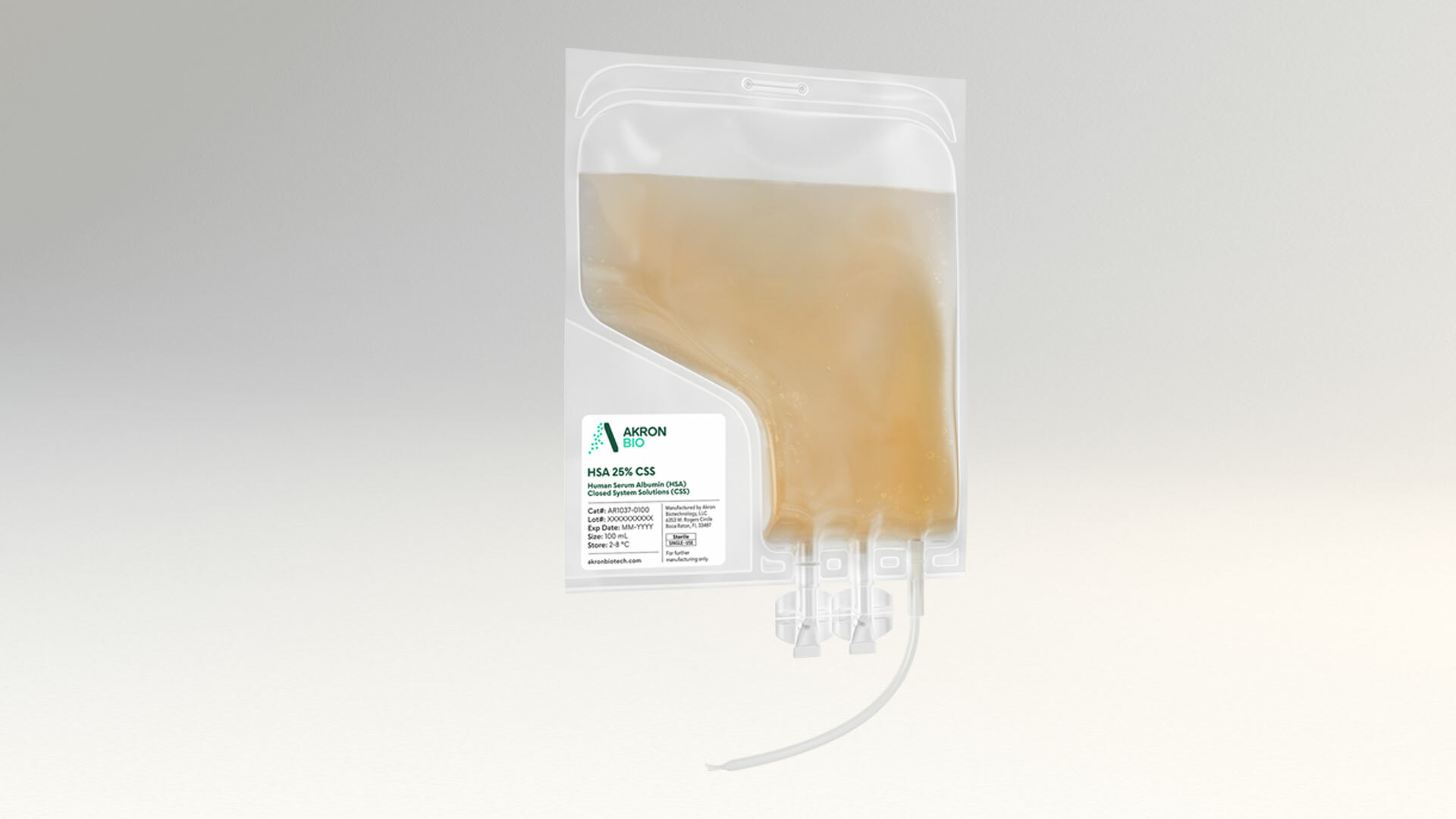
Human Plasma Derived Products
Akron’s proven human plasma-derived products deliver safety and performance backed by robust and redundant supply. Because you can’t afford to compromise.
Our human plasma-derived products are engineered specifically with our advanced therapy customer’s needs in mind. We've designed each of these products to deliver optimal cell growth and viability within cGMP manufacturing processes, never compromising on safety and lot-to-lot consistency.
These products are backed
with comprehensive manufacturing and pathogen safety documentation that aims to
meet the commercial regulatory requirements of leading health authorities within
the US, EU, and Asia.
Custom Services
Custom size and configuration vials, syringes, bags and more. We manufacture custom recombinant proteins, media and buffers for cell and gene therapy products based on your specifications, all under cGMP guidelines.
Capitalizing on our established platform for manufacturing cGMP-compliant clinical and commercial grade ancillary products. Our broad expertise and vision are highly sought after, particularly for challenging projects which demand carefully considered approaches.