Posters & Publications
Posters


Dual Cation Exchange Coupled with Multi-Modal Chromatography Allows the Production of High-Purity and Low Residuals cGMP Grade Cas9 Nuclease for Cell Therapy Applications
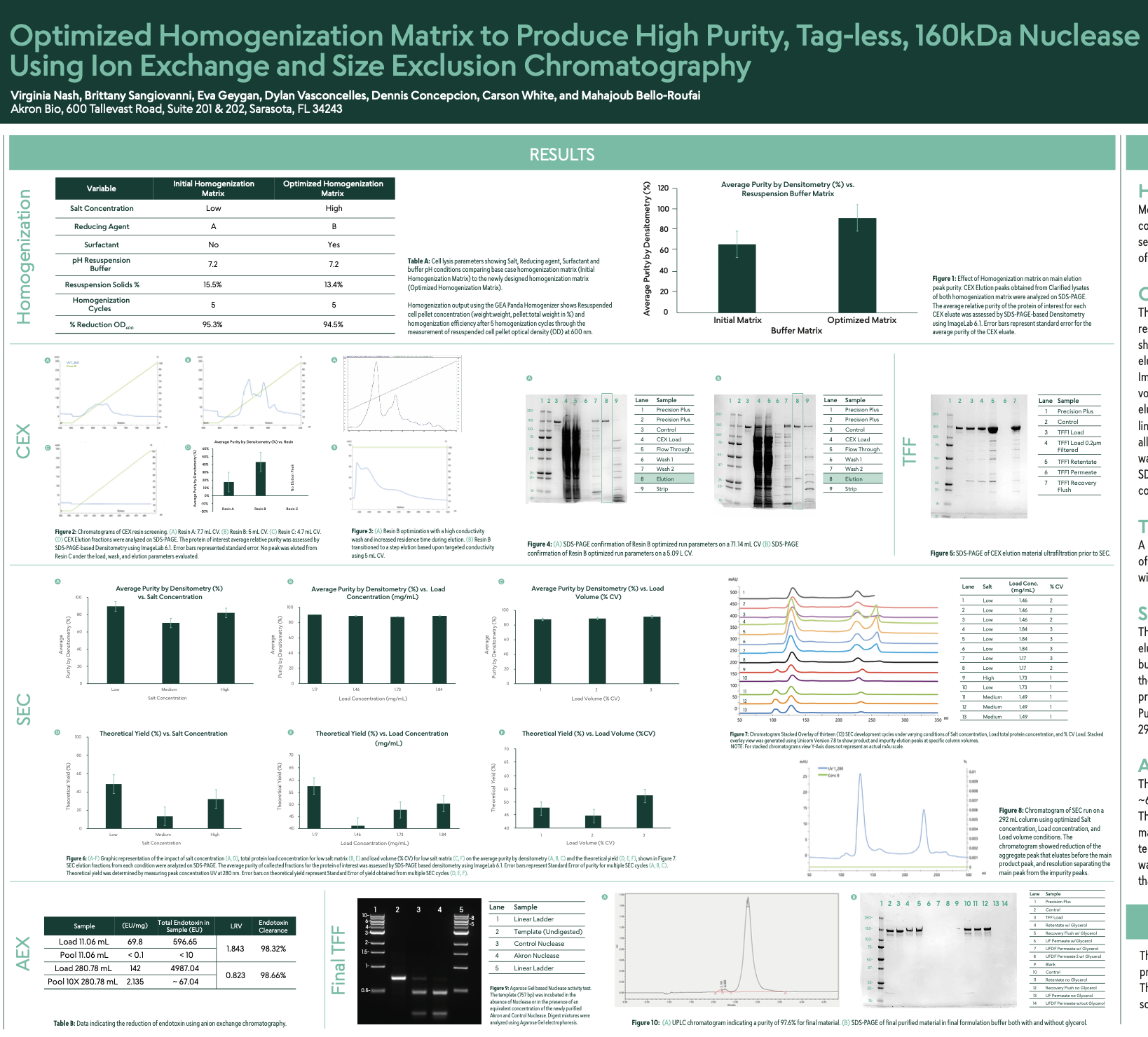
Optimized Homogenization Matrix to Produce High Purity, Tag-less, 160kDa Nuclease Using Ion Exchange and Size Exclusion Chromatography
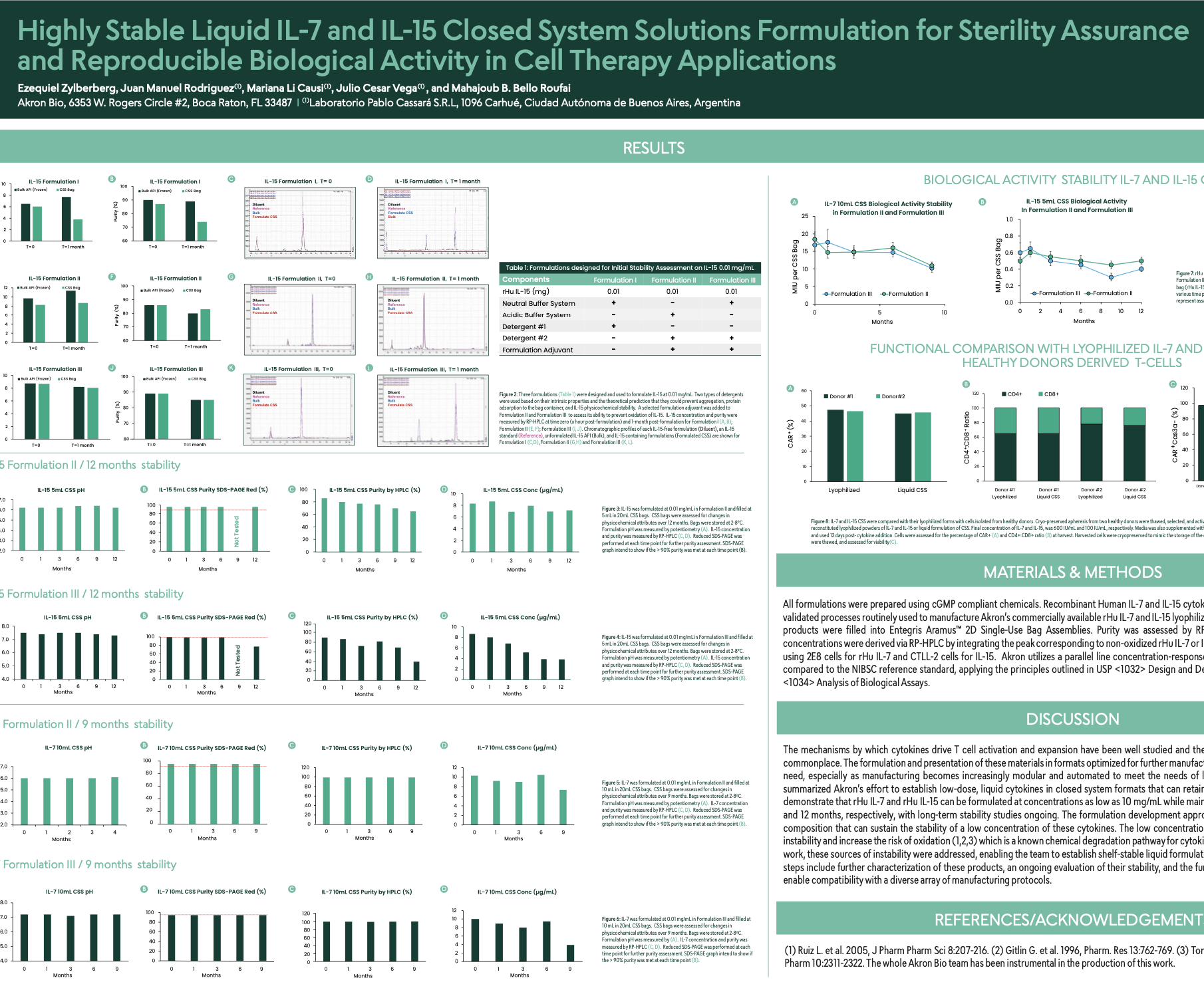
Highly Stable Liquid IL-7 and IL-15 Closed System Solutions formulation for Sterility assurance and Reproducible Biological Activity in cell therapy application
-
1
-
2
Publications
Nathan D. Frank, Ezequiel Zylberberg, Mahajoub Bello Roufai, Stuart L. Gibb, and Mindy M. Miller. Good Manufacturing Practice-Grade Fibronectin for Hollow-Fiber Bioreactor Cell Manufacture: A Mesenchymal Stromal Cell Case Study. Cytotherapy, December 6, 2024.
Jonian Grosha, Jun-Hung Cho, Shannon Pasley, Peter Kilbride, Claudia Zylberberg, and Marsha W. Rolle Engineered Test Tissues: A Model for Quantifying the Effects of Cryopreservation Parameters. ACS Biomaterials Science & Engineering, October 6, 2023, DOI: 10.1021/acsbiomaterials.3c00752
Burke CJ, Zylberberg C. Sources of Variability in Manufacturing of Cell Therapies. Regenerative Engineering and Translational Medicine, 2019, DOI 10.1007/s40883-019-00130-5
Harrison RP, Zylberberg E, Ellison S, Levine BL. Chimeric antigen receptor–T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy, 2019, In Press.
El Assal, L. Abou‐Elkacem, A. Tocchio, S. Pasley, S. Matosevic, D. L. Kaplan, C. Zylberberg, U. Demirci. Bioinspired Preservation of Natural Killer Cells for Cancer Immunotherapy. Adv. Sci.2019, 1802045.
Bruce CJ, Guojun B, Centanni JM, Davis MD, Dobson J, Hare JM, Fields GB, Jove R, Kenyon N, Kahn A, March K, Matosevic S, Mahmood A, Pepine CJ, Ricordi C, Shapiro SA, Zylberberg C, Zylberberg E. Regenerative Medicine in the State of Florida: Letter Outlining the Florida Organization for Regenerative Medicine. Stem Cells Translational Medicine, 2018, 7(7): 511-512.
National Academies of Sciences, Engineering, and Medicine. 2017. Navigating the Manufacturing Process and Ensuring the Quality of Regenerative Medicine Therapies: Proceedings of a Workshop. Washington, DC: The National Academies Press.
Zylberberg C and Matosevic S. Bioengineered liposome-scaffold composites as therapeutic delivery systems. Therapeutic Delivery, 2017, 8(6):425-445.
Zylberberg C, Gaskill K, Pasley S, Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Therapy, 2017, 24(8):441-452.
Haddock R, Lin-Gibson S, Lumelsky N, McFarland R, Roy K, Saha K, Zhang J, Zylberberg C. Manufacturing Cell Therapies: The Paradigm Shift in Health Care of This Century. 2017, National Academy of Medicine, 1-13.
Buckler L, Kunkel E, Thompson M, Ehrhardt R. Technological developments for small-scale downstream processing of cell therapies. 2016, Cytotherapy, 18(3):301-306.
MForesight: Alliance for Manufacturing Foresight. Biomanufacturing Technologies for Regenerative Medicine. 2016.
Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. 2016, Drug delivery, 23(9):3319-3329.
Solomon J, Csontos L, Clarke D, Bonvhadi M, Zylberberg C, McNiece I, Kurtzberg J, Bell R, Deans R. Current perspectives on the use of ancillary materials for the manufacture of cellular therapies. 2016, Cytotherapy, 18(1):1-12.
Knapinska A, Amar S, He Z, Matosevic S, Zylberberg C, Fields G. Matrix metalloproteinases as reagents for cell isolation. Enzyme and Microbial Technology, 2016, 93-94:29-43.
Porat Y, Abraham E, O Karnieli, Nahum S, Woda J, Zylberberg C. Critical elements in the development of cell therapy potency assays for ischemic conditions. 2015, Cytotherapy, 17(7):817-831.
Abbott S, MacKay G, Durdy M, Solomon S, Zylberberg C. Twenty years of the International Society for Cellular Therapies: the past, present and future of cellular therapy clinical development. 2014, Cytotherapy, 16(4):S112-S119.
Assal RE, Guven S, Gurkan UA, et al. Bio-inspired Cryo-ink Preserves Red Blood Cell Phenotype and Function during Nanoliter Vitrification. 2014, Advanced materials, 26(33):5815-5822.