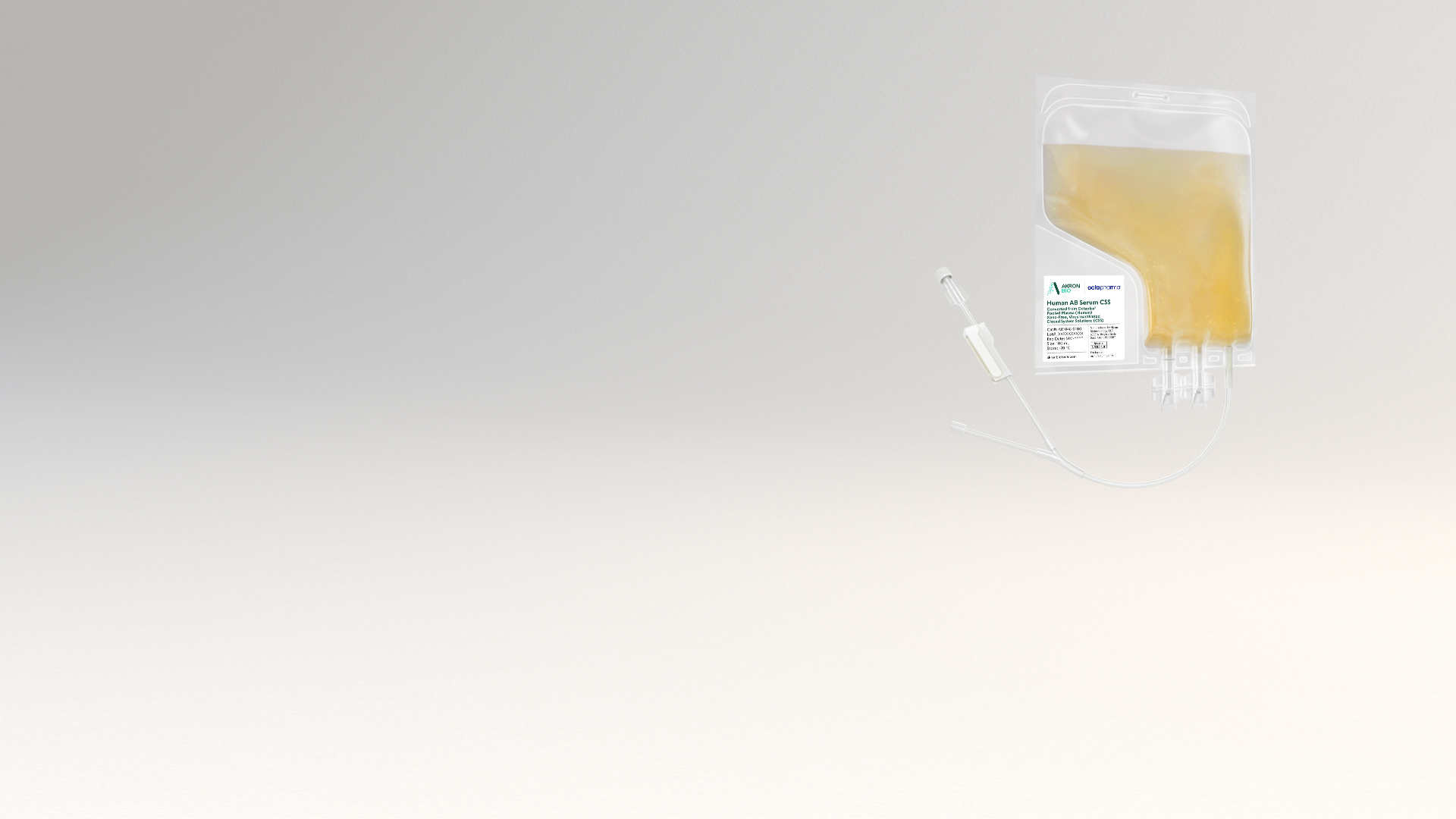
cGMP Human AB Serum CSS,
Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated
Our cGMP AB serum delivers the best in safety and consistency.
Akron’s Human AB Serum, Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated (viHABS) Closed System Solutions (CSS)™ is manufactured, tested, and released following relevant cGMP guidelines for blood-derived ancillary materials.
Raw material donor selection ensures that the product is devoid of antibodies for A and B blood antigens, therefore minimizing immune reactivity in cell culture. No animal-derived components are used in the manufacture of this product. Virus-inactivated plasma is used for all human-derived components. This product is considered a comparable and superior alternative to fetal bovine serum or other animal-based products. By leveraging Octaplas®, a pharmaceutically licensed virus inactivated and prion-ligand treated plasma, as a raw material, Akron is now offering a high quality, virus inactivated human AB serum product with greater batch-to-batch consistency and a unique safety profile.
The viHABS CSS product is packaged in a sterile single-use bag with weldable tubing, allowing for easy incorporation into modern closed-system cell culture bioprocessing protocols. viHABS CSS increases safety and ease of use by allowing for the introduction of supplement material into culture media in a fully contained manner. The final product undergoes Endotoxin, Mycoplasma, and Sterility testing. See product Packaging features below.
Donor Eligibility
- Pharmaceutically licensed, pooled plasma from US licensed plasma donation centers
- Collection complies with WHO Technical Report Series 840: “The Collection, Fractionation, Quality Control, and uses of Blood and Blood Products
- Nucleic acid virus testing (NAT) during multiple stages of manufacturing (HIV, B19, HAV, HBV, HCV, HEV)
- Donor screening and virus testing per 21 CFR 610.40
- Type AB donations devoid of antibodies for A and B blood antigens
Plasma Source
- Octaplas® is an FDA-approved, sterile, pyrogen-free, frozen, S/D treated, prion-ligand treated, pooled human plasma
- Solvent Detergent (S/D) treatment for inactivation of enveloped viruses
- Immune Neutralization for inactivation of certain non-enveloped viruses
- Affinity Chromatography intended to reduce prion proteins
- Sterile microfiltration minimizes the presence of bacteria and parasites
Manufacturing
- No animal-derived materials are used in the manufacture of this product
- Sterile microfiltration followed by aseptic filling
Packaging
- Sterile bag chamber – Ethylene-vinyl acetate (EVA) for inert bio-reactivity and increased flexibility
- Weldable polyvinyl chloride (PVC) 6” outlet tubing (2.5 mm ID x 4.1 mm OD) with female Luer adapter
- End of inlet tubing sealed using controlled radiofrequency or heat
- Two twist-off spike ports allowing for various attachments and adapters to fit your purpose
- Tubes and ports are welded into the bag chamber eliminating potential failure points
- All primary packaging is plasticizer free
- Primary packaging materials extensively validated, controlled, and qualified to ensure a consistent experience
Quality
- Relevant cGMP guidelines used in manufacture, testing, and release
- USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
- ISO 20399:2022, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products
Stability
- 24-month shelf life
- Store at -20 °C
- Transport on dry ice
- Avoid repeated freeze-thaw cycles
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
• Appearance
• pH
• Osmolality
• Mycoplasma
• Bacterial Endotoxins
• Sterility
• Total Protein and Comprehensive Metabolic Panel
1. Why use Human AB Serum, Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated (viHABS) Closed System Solutions (CSS)™?
Human AB Serum is used as a media supplement in cell culture. This product is considered a comparable alternative to fetal bovine serum or other animal-based products. Raw material donor selection ensures that the product is devoid of antibodies for A and B blood antigens, therefore minimizing immune reactivity in cell culture. We leverage Octaplas® (pharmaceutically licensed virus inactivated and prion-ligand treated plasma) as the raw material, offering greater batch-to-batch consistency and a unique safety profile.
Akron’s novel viHABS CSS formulation and sterile bag packaging are ideal for closed-system commercial cell therapy manufacturing, allowing for the introduction of supplement material into culture media in a fully contained manner.
2. What are the recommended storage conditions for viHABS CSS?
We recommend storing this product at -20 °C. If serum content is not being used all at once, we suggest aliquoting into the desired volume and storing at -20 °C until needed. Avoid repeated freeze-thaw cycles.
3. What are the shipping conditions for viHABS CSS?
This product ships with dry ice.
4. What is the shelf-life for Human AB Serum, Converted from Octaplas®, XF, VI, CSS?
These products have a shelf life of 24 months after the date of manufacture under recommended controlled storage conditions.
5. What is the thawing procedure for viHABS CSS?
Thaw at 2-8 °C for 24 hours, then allow to reach room temperature. If further warming is required, use a controlled 37 °C environment to bring up to temperature. Avoid multiple freeze-thaw cycles, prolonged exposure to 37 °C, and temperatures above 37 °C.
6. Do particulates ever form after thawing?
Precipitates will form in certain units of serum during the thawing process. This is normal and common behavior for serum products and it will not negatively affect cell culture. These precipitates do not negatively impact the safety or quality of the material. This product is sterile filtered before filling and every lot is tested for mycoplasma and sterility per USP.
7. Should I heat inactivate the Human AB Serum before use?
Our Human AB Serum product is sold ready to use directly as a supplement to your culture medium, but we have seen increased performance with certain cell lines after a heat treatment process is applied to the product before use. If performing this, we recommend a temperature of about 56 °C for 30 minutes; inactivation for a longer time may have a negative effect on cell growth due to denaturing of proteins and growth factors.
8. Where is the raw material sourced?
viHABS CSS is converted from Octaplas®. Only plasma from US donors (frozen within 8 hours of collection) is used to manufacture Octaplas® and ultimately the Human AB Serum. For Octaplas®, individual donor identification, registration, and education takes place in FDA-licensed centers which comply to the requirements for the collection of source plasma as specified in “The Collection, Fractionation, Quality Control, and uses of Blood and Blood Products,“ published by the WHO Technical Report Series 840. This allows for traceability down to the single donation unit, if needed.
9. How are the raw material donations screened?
There is a deferral check and questionnaire at each donation, as well as a physical assessment, per 21 CFR 630.10. Each plasma unit used as raw material is virus tested per 21 CFR 610.40 at the time of collection and found negative or non-reactive for Hepatitis B surface Antigen (HBsAg), antibodies to Human Immunodeficiency Virus (HIV)-1/2 (anti-HIV-1/2) and antibodies to Hepatitis C Virus (anti-HCV). HBV and HIV-1/2 serological tests are also performed on each plasma pool. Nucleic Acid Testing (NAT) is also performed at different manufacturing stages for HIV, B19V, HAV, HBV, HCV and HEV.
10. What process is used for viral inactivation?
Viral inactivation of enveloped viruses is accomplished via solvent detergent (S/D) treatment (see Technical Overview).
11. What are the advantages of S/D treatment versus other inactivation methods currently used?
S/D treatment has long been a standard and robust virus inactivation process for plasma products. S/D and pH inactivation are generally effective against enveloped viruses. Heat inactivation, irradiation, chromatography and filtration methods have been shown to have some effect on non-enveloped viruses, as well as enveloped viruses. The viral safety for plasma products should be validated with a panel of characterized viruses, and pathogen safety studies should be incorporated for all relevant steps of the manufacturing process. A combination of these methods are used to reduce the overall risk of adventitious viruses and bloodborne pathogens in plasma products.
12. Are non-enveloped viruses inactivated also?
S/D treatment is not effective against non-enveloped viruses. However, the presence of standardized levels of neutralizing antibodies in the Octaplas® plasma pool along with virus load control by NAT screening minimizes the risk of transmitting non-enveloped viruses such as HAV, and parvovirus B19V (see Technical Overview).
13. Is there S/D agent remaining in the plasma that might affect cell growth?
In the Octaplas® manufacturing process, the S/D reagents are removed by sequential oil and solid phase extraction procedures. Final levels of residual S/D agents in Octaplas® are below FDA-specified limits for product release. These remaining levels of S/D agents are below what has been found to impair cell growth.
14. What is the difference between plasma and serum?
Whole blood is composed of plasma, red and white blood cells, and platelets. Plasma is the part of blood that carries nutrients, antibodies and hormones throughout the body. It contains serum and clotting factors. Serum is plasma without clotting factors.
15. Which cell types are suitable?
Akron’s Human AB Serum, Converted from Octaplas®, XF, VI can be used as a replacement in any cell culture protocols that currently use human AB serum or fetal bovine serum (FBS). Optimization may be required.
16. Is the performance comparable to competitor products / FBS?
Cell proliferation and viability are comparable to other serum products on the market, including FBS, while offering increased safety associated with the raw materials and manufacturing process (ask us for supporting data).
17. Do you have an SDS for this product?
Yes, an SDS is available upon request.
18. Is this an injectable-grade or clinical-grade material?
No, this product is not for direct use in humans. See intended use below.
19. What is the intended use for the product?
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
20. What packaging options are available?
Akron’s AB Serum CSS comes packaged in a sterile bag chamber made from Ethylene-vinyl acetate (EVA) for inert bioreactivity and increased flexibility. The 6” outlet tubing on the distal end of the Y connector is made from weldable polyvinyl chloride (PVC) (2.5 mm ID x 4.1 mm OD). This product can be customized, with different packaging materials and/or fill volumes available under contract.
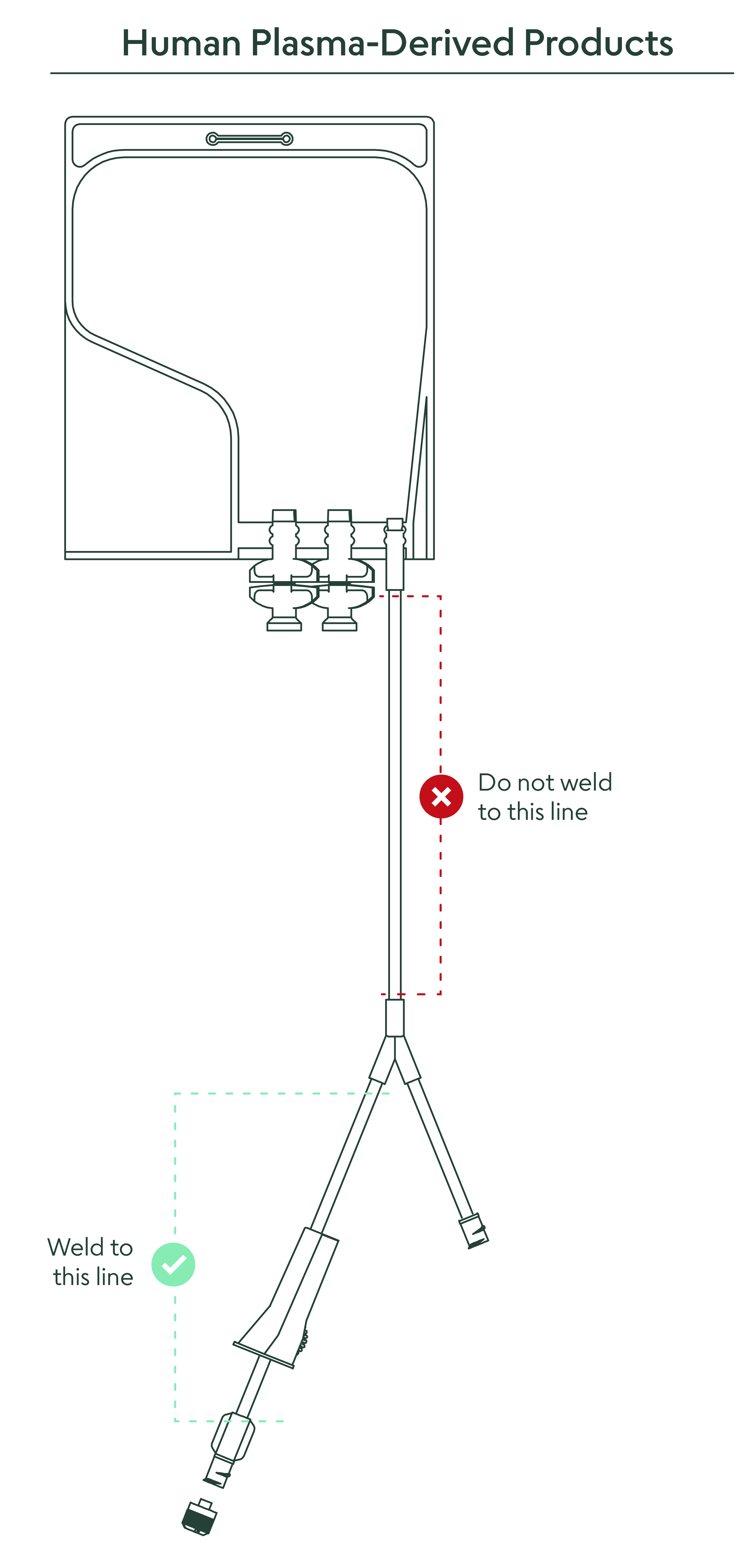
21. What closed connection options are available to remove the material from the bag?
It is recommended to weld directly to the 6” PVC outlet tubing. A direct connection via a female Luer lock connector is available on the outlet tube. There are also two twist-off spike ports for entry with a spike adapter if desired (for full instructions, see “Methods of Use” document).
Bags (60 mL) Cat. # AR1048-0060
Bags (100 mL) Cat. # AR1048-0100