Recombinant Human IL-2
Our cGMP IL-2 is available in a lyophilized vial, liquid syringe, or our innovative Closed System Solutions (CSS)™ single-use bags
Akron’s Recombinant Human Interleukin-2 (rHu IL-2) products are manufactured following all relevant cGMP guidelines for ancillary materials and are supported by a Type II Master File (MF) on file with the FDA and an MF Type I on file with Health Canada, which can be referenced during your drug or biologic application process.
Available in various aliquots and formats
Akron offers its IL-2 in various formats and aliquots to better suit our customer’s needs. Whether the need is for pre-clinical use or for a commercial-grade product, we provide the sizes and formats necessary.
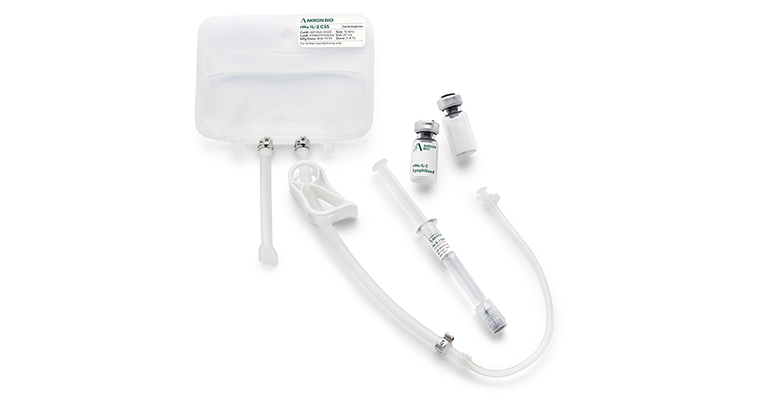
Activate and expand T cells, and NK cells with Akron’s IL-2
Akron’s rHU IL-2 reliably promotes the proliferation and differentiation of CAR T cells, T cells, TCR T cells, Tregs, TILS and natural killer cells (NK cells).
Contact our team of experts to test Akron’s rHU IL-2 today