cGMP rHu IL-2 Prefilled Syringe
Novel cGMP liquid formulation & simplified packaging for convenience and safety
Akron’s Recombinant Human Interleukin-2 (rHu IL-2) products are manufactured following all relevant cGMP guidelines for ancillary materials and are supported by a Type II Master File (MF) on file with the FDA and an MF Type I on file with Health Canada which can be referenced during your drug or biologic application process. Our rHu IL-2 amino acid sequence is identical to Proleukin® (aldesleukin), and its functional similarity in T Cell expansion has been evaluated and confirmed (see page 3). Akron’s rHu IL-2 is a single chain, 15.3 kDa, non-glycosylated lymphokine analog expressed in E. coli, containing 132 amino acids.
It is purified in a pharmaceutical facility without the use of histidine tags and nickel columns. Sterile filtration with aseptic filling are performed with Endotoxin and Sterility testing performed per USP/EP on the final product. The novel liquid formulation and syringe packaging increase safety and ease of use by eliminating the reconstitution step during manufacture. The liquid syringes are available in 1 mg aliquots.
Active Substance
• Amino acid sequence identical to Proleukin® / aldesleukin
• Carrier protein-free formulation
• E. coli expression system
• All raw materials are compliant, controlled, and traceable under Akron’s Quality Management System (QMS)
Manufacturing
• Type II eCTD MF (#026152) on file with the FDA and MF Type I (#e250089) on file with Health Canada
• Tag-free pharmaceutical processing
• Gram-scale production capacity
Packaging
• Type I borosilicate glass syringe compliant per USP <660> Containers-Glass
• Luer-lock tip connector, for a tight seal and quick disconnect, compliant per USP <381> Elastomeric Closures for Injections
• Plunger stopper provides an effective barrier against extractables and leachables
Quality
• Relevant cGMP guidelines used in manufacture, testing, and release
• USP <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
• EP 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products
• ISO 20399:2022, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products
• High Purity, Low Endotoxin - Endotoxin and Sterility testing per USP/EP
Stability
• 24-month shelf life
• Store at 2-8 °C
• Transport with cold packs
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
NOTICE: Catalog #AK9984 is patent pending.
• Appearance
• pH
• Protein Concentration
• Western Blot
• Reducing SDS-PAGE
• Impurities (Coomasie staining)
• Impurities (Silver staining)
• Biological Activity
• Specific Activity
• Bacterial Endotoxins
• Sterility
Akron rHu IL-2 vs Proleukin® - pSTAT5 Expression

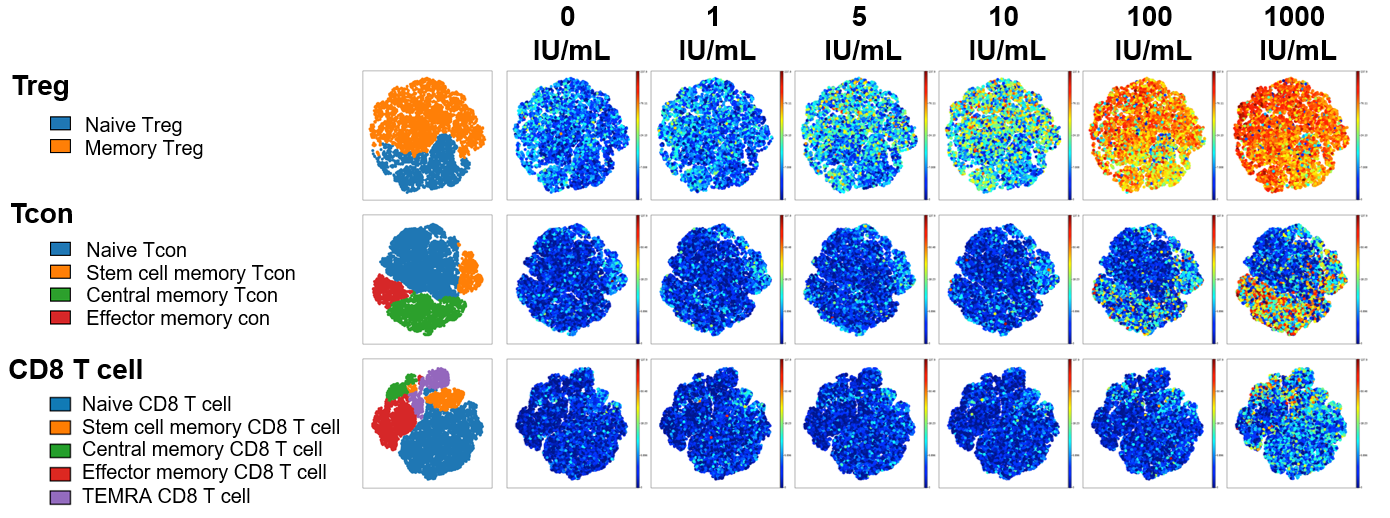
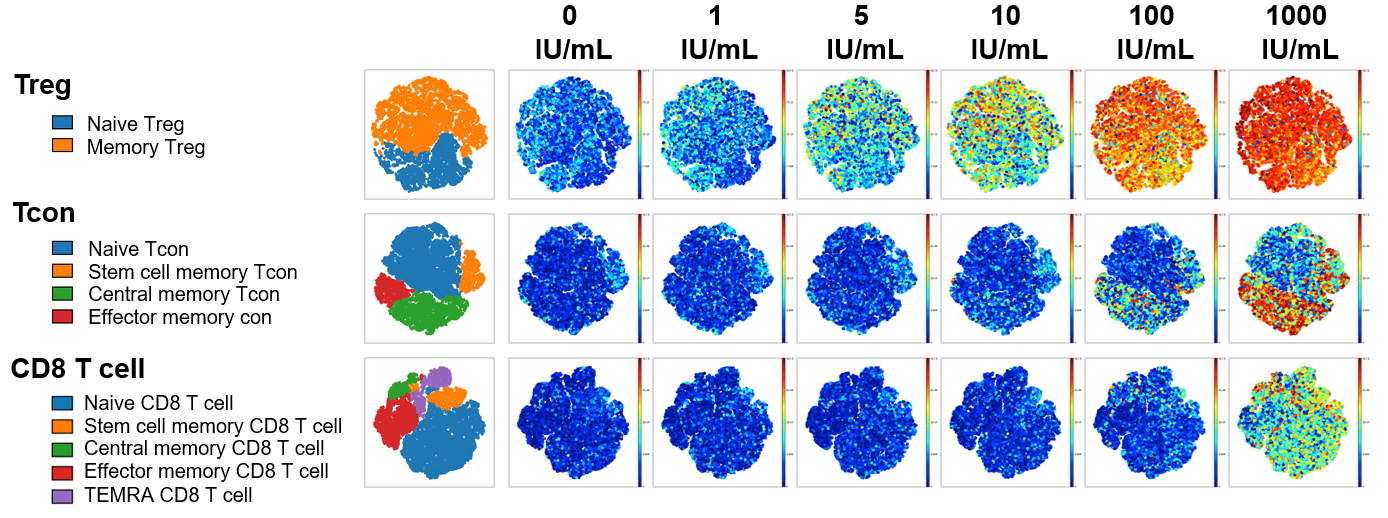
Figure 1: To examine IL-2 signaling, freshly isolated PBMC from healthy donors were stained with surface antibodies targeting 21 different protein markers prior to in vitro stimulation with IL-2. Single mass cell cytometry was used to compare the effect of Akron’s rHu IL-2 (top) against Proleukin® (bottom) on the expression of pSTAT5, pSTAT3, & pSTAT1 in T cell subsets. (pSTAT5 example shown above).
When results were summarized for six healthy donors, T cell stimulation in vitro by Proleukin® and Akron rHu IL-2 were indistinguishable.
This study was done in collaboration with the Dana-Farber Cancer Institute; study and poster available upon request.
1. Why use Akron’s Recombinant Human Interleukin-2 (rHu IL-2) Prefilled Syringe?
The novel liquid rHu IL-2 formulation and syringe packaging increase safety and ease of use by eliminating the reconstitution step during manufacture. Akron’s Recombinant Human Interleukin-2 (rHu IL-2) is manufactured following all relevant cGMP guidelines for ancillary materials and is supported by a Type II Master File (MF) with the FDA and a MF Type 1 with Health Canada. It is purified in a pharmaceutical facility without the use of histidine tags and nickel columns. Sterile filtration with aseptic filling and lyophilization are performed in-house with Endotoxin and Sterility testing performed per USP/EP on the final product. Our rHu IL-2 amino acid sequence is identical to Proleukin® (aldesleukin), and its functional similarity in T cell expansion has been evaluated and confirmed (see Product Page).
2. What are the recommended storage conditions for Akron’s rHu IL-2?
We recommend storing this product at 2-8 °C.
3. What is the white precipitate inside the syringe that is observed during the recommended storage conditions?
During storage at 2-8 °C, a white precipitate appears inside the syringe as a result of a reversible interaction between the rHu IL-2 protein and the excipients at this temperature. The white precipitate disappears after a few minutes at room temperature and has no relevant impact on the strength, purity, or biological activity of Akron’s rHu IL-2. Once the precipitate has reached dissolution, a colorless, clear liquid should be observed within the syringe, which is now ready to use. Warming up the syringe with the hands, rolling the syringe back and forth slowly between them to gently swirl the content may accelerate the process.
NOTE: DO NOT USE THE SYRINGE UNTIL THE PRECIPITATE COMPLETELY DISSOLVES AND IS NO LONGER VISIBLE.
4. What are the shipping conditions for Akron’s rHu IL-2 Prefilled Syringe?
This product ships with cold packs.
5. What is the shelf-life for Akron’s rHu IL-2 Prefilled Syringe?
This product has a shelf life of 24 months after the date of manufacture under recommended controlled storage conditions.
6. Do you have any excursion stability data available?
This product has been shown to meet original release specifications when stored at 25 °C and 60 % Relative Humidity for 15 days and when stored at -20 °C for 7 days.
7. What organism is used to express Akron’s rHu IL-2?
This product is expressed in E. coli.
8. How is Akron’s rHu IL-2 different than native IL-2?
Akron’s rHu IL-2 is not glycosylated because it is derived from E. coli and has an identical amino acid sequence to Proleukin® (aldesleukin), which differs from the native IL-2 amino sequence in the following ways: Akron’s rHu IL-2 does not have an N-terminal alanine, and it has a serine substituted in place of a cysteine at position 125. It is likely that the aggregation state is also different than native IL-2.
9. What is the molecular weight of Akron’s rHu IL-2?
Akron’s rHu IL-2 was characterized by liquid chromatography-electrospray ionization-tandem mass spectrometry analysis and was found to have an observed molecular mass of 15,319 Da. Every batch of product is released using a reducing SDS-PAGE molecular weight assay to directly compare against a reference standard.
10. Do you have a Master File (MF) available for this product?
Yes, our rHu IL-2 has a Type II eCTD MF (#026152) on file with the FDA and an MF Type I (#e250089) on file with Health Canada. We can provide you a Letter of Authorization that will permit these agencies to refer to the information on file in support of your submissions.
11. Does this product have a TSE/BSE statement?
Yes, a TSE/BSE statement is available upon request for this product.
12. Are animal-derived materials used in the manufacture of this product?
All raw materials, components, sub-components, and consumables used in the manufacturing of this product are either animal-free or in compliance with EMEA/410/01 rev. 3. Please see the BSE-TSE Statement.
13. Is virus and pathogen inactivation included in the manufacturing process?
No, because viruses that infect bacteria (bacteriophages) do not pose a known threat to human cells. Virus reduction manufacturing steps are usually not included when purifying material from a bacterial host, as is the case with Akron’s rHu IL-2. We use an E. coli based expression system that does not require additional virus reduction.
14. What safety testing is done on this product?
Every lot of final product is tested and released, with specifications and methods per USP/EP, for both Endotoxin (USP <85> / EP 2.6.14) and Sterility (USP <71> / EP 2.6.1).
15. Which cell types are suitable?
Akron’s cGMP-compliant rHu IL-2 can be used to promote the activation and proliferation of numerous immune cell types, including CAR-T cells, TCR-T cells, Tregs, TILs, NK cells, CIK cells, B cells, monocytes, and macrophages.
16. Do you have an SDS for this product?
Yes, an SDS is available upon request for this product.
17. How does Akron measure activity for rHu IL-2?
Akron uses a validated leukocyte proliferation assay to report activity. The rHu IL-2 induced proliferation of T lymphocyte HT-2 cells is verified against the World Health Organization (WHO) 2nd international standard by the National Institute for Biological Standards and Control for Interleukin-2 (NIBSC 86/500). Akron uses a parallel-line concentration-response model to calculate a relative potency, per guidelines set forth in USP <1032> and USP <1034> (see Technical Overview).
18. Is the performance comparable to competitor rHu IL-2 products?
The specific activity (MIU/mg) of Akron’s rHu IL-2 batches have historically been comparable to the specific activity of the current international standard (NIBSC 86/500) for IL-2, known to be approximately 13.73 MIU/mg. Akron’s rHu IL-2 amino acid sequence is identical to Proleukin® (aldesleukin), and its functional similarity in T Cells has been evaluated and confirmed (see Product Page Data).
19. What is the intended use for the product?
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
20. What packaging options are available?
Akron’s rHu IL-2 Prefilled Syringe comes packaged into a Type I borosilicate glass syringe with a rubber stopper and a Luer-lock tip. Closed-system bags for Akron’s rHu IL-2 liquid formulation are also available (inquire for more details).
DO NOT USE UNTIL THE PRECIPITATE COMPLETELY DISSOLVES AND IS NO LONGER VISIBLE.
During storage at 2-8 °C, a white precipitate appears inside the syringe. This is the result of a reversible interaction between the rHu IL-2 protein and excipients at this temperature, which brings about no relevant changes in strength, purity, or biological activity of Akron’s rHu IL-2. The precipitate disappears after a few minutes at room temperature. Warming up the syringe with the hands, rolling the syringe back and forth slowly between them to gently swirl the content may accelerate the process. Once the precipitate has reached dissolution, a colorless, clear liquid should be observed within the syringe, which is now ready to use. Please ensure the white precipitate, if present, completely dissolves and is no longer visible prior to use.
IL-2 plays a major role in both upregulating and downregulating the body’s immune response. It is critical for the homeostasis and differentiation of many immune cell types and is involved in the immune system’s ability for self-tolerance. The pleiotropic nature of cytokines is especially diverse in IL-2 due to its signal being transduced by at least three different primary signaling pathways. The trimeric IL-2 receptor protein (IL-2R) shares an identical subunit with the IL-7, IL-15, and IL-21 receptor proteins and activates some of the same signal transduction mechanisms. Akron’s cGMP-compliant rHu IL-2 can be used to promote the activation and proliferation of numerous immune cell types, including CAR-T cells, TCR-T cells, Tregs, TILs, NK cells, CIK cells, B cells, monocytes, and macrophages.
Aliquot Sizes & Formats
Liquid Syringe (1 mg) Cat. # AK9984-1000