Ensure Sterility and Enable Scalable Manufacturing
Our cGMP cytokines in single-use bags are designed for convenience and performance. Stable at 2–8°C for up to one year, our CSS cytokines enable plug-and-play integration into any immune cell manufacturing system, no thawing required.
Backed by FDA Drug Master Files, our CSS cytokines support smooth regulatory submissions and scale-up from preclinical to commercial production.
Currently available in IL-2, IL-7, and IL-15, with flexible fill volumes and concentrations to meet your specific manufacturing needs.
Performance-Ready for Closed System Workflows
Compatibility
No platform limitations. Compatible with your existing immune cell manufacturing system.
Weldable Tubing
Each bag is designed with weldable PVC tubing, enabling direct sterile connection to media bags or bioreactor input systems.
Weld & run
No need for reconstitution or transfer steps. Akron’s CSS format cuts down on technician time and reduces human error in critical workflows.
Ready-to-Use
No need to thaw, stable at 2–8 °C. Go directly from the fridge to your cell culture.
Closed System Solutions (CSS)™ Cytokines Now Available
Say goodbye to wasted time and human error
Our liquid cytokines offer the quality, consistency, and reliability needed to support your immune cell therapy workflows. Simplify your process with ready-to-use cytokines in weldable bags.
- Reduces unnecessary complexity by eliminating reconstitution
- Multiple tubing options allow for seamless integration into closed systems
- Reduces human labor and error

Reducing the Complexity, Time, Risk, and Cost
We designed our CSS product line from the ground up to address the evolving challenges in cell manufacturing faced by T cell therapeutic developers. Read our blog post to learn why we created the CSS line—and how developers are now benefiting from it.
Functional Comparison with Lyophilized II-7 And II-15 Using Healthy Donors Derived T-Cells
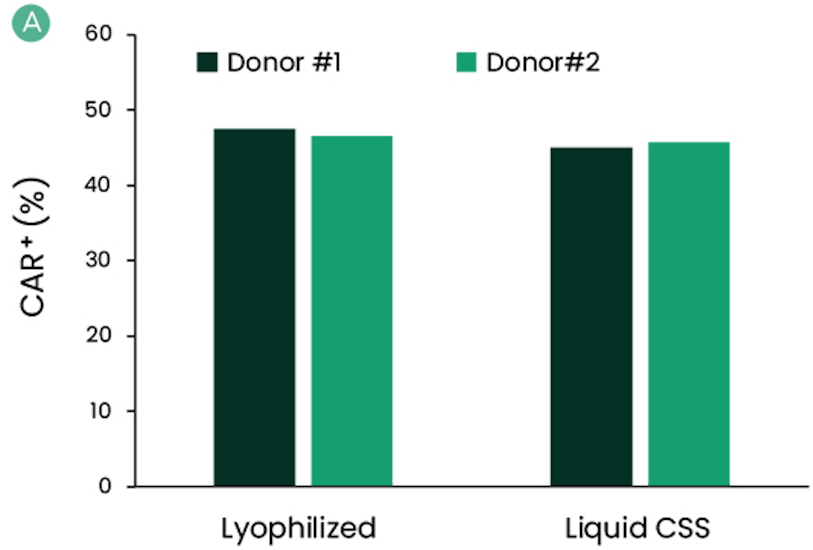
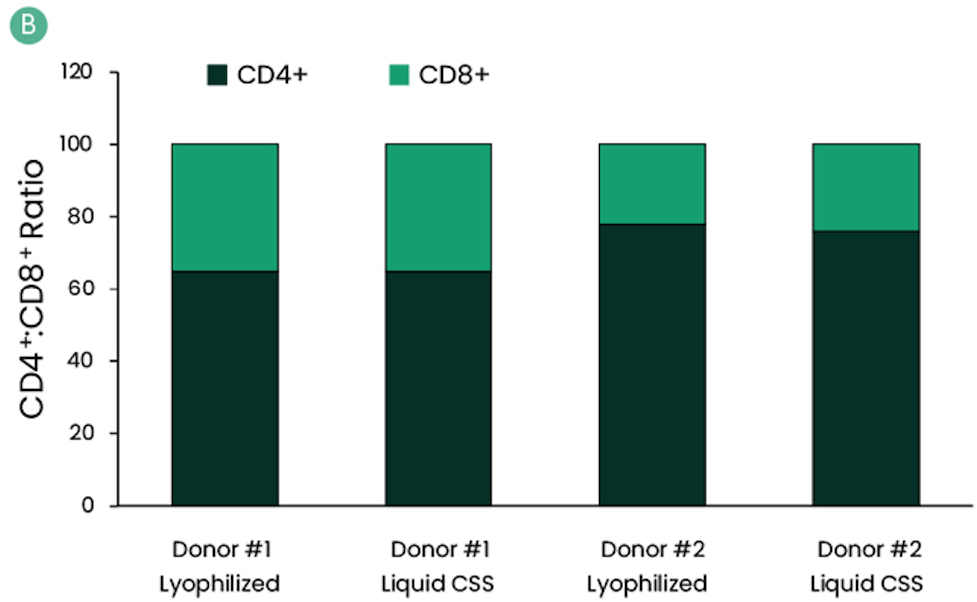
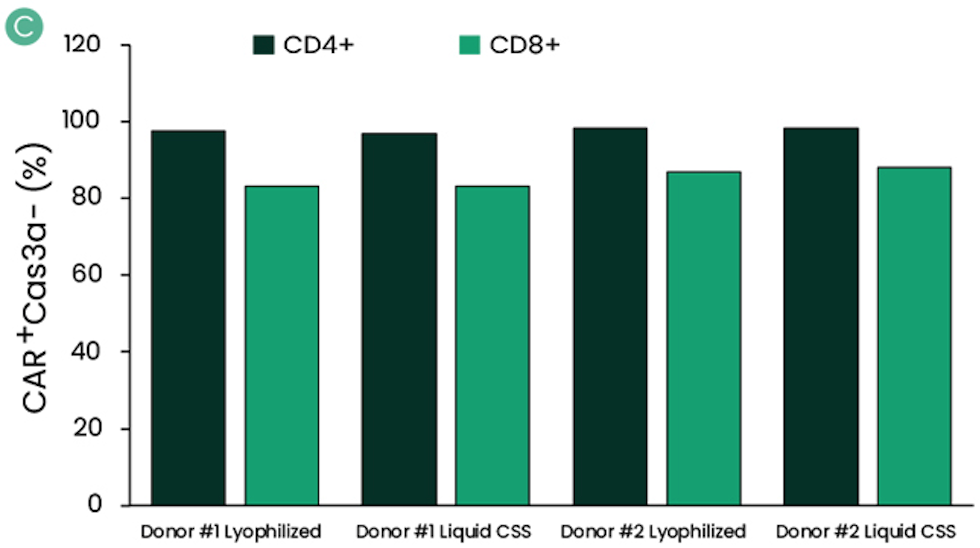
Figure: IL-7 and IL-15 CSS were compared with their lyophilized forms with cells isolated from healthy donors. Cryo-preserved apheresis from two healthy donors were thawed, selected, and activated. Culture media were supplemented with reconstituted lyophilized powders of IL-7 and IL-15 or liquid formulation of CSS. Final concentration of IL-7 and IL-15 was 600 IU/mL and 100 IU/mL, respectively. Media was also supplemented with 100 IU/mL of IL-2. Prepared media were stored at 2–8°C and used 12 days post-cytokine addition. Cells were assessed for the percentage of CAR+ (A) and CD4+:CD8+ ratio (B) at harvest. Harvested cells were cryopreserved to mimic the storage of the drug product. Several days after cryopreservation, cells were thawed and assessed for viability (C).
