Reducing the Complexity, Time, Risk, and Cost of Media Formulation in Cell Therapy Manufacturing
Cell Therapy Manufacturing Challenges
Treatment of certain hematological cancers has been vastly improved with the advent of chimeric antigen receptor (CAR) T cell therapies. Although these cell therapies have demonstrated efficacy, only a small percentage of people stricken with these cancers have received CAR-T treatments. In addition to the complex orchestration required to manufacture these advanced therapies and the limited number of clinical sites capable of administering them, the greatest barrier to widespread adoption is their high cost, which can reach $500,000 or more per treatment1.
The solution to this limited availability will be multifaceted and require innovation on a variety of fronts from technology advances to new payment paradigms. Critically, these engineered cell therapies need reduction of cost of goods (COGs) through next generation raw materials and the development of more robust production processes that are simpler, more modular, and further streamlined.
Simplifying the use to increase the reliability of raw materials is one important area for such innovation. Traditionally, media supplements such as cytokines and growth factors have been supplied as lyophilized powders that require reconstitution. This is due to the fact that these molecules tend to rapidly degrade in solution. Reconstitution and media formulations can take upwards of one or more hours to complete due to the need to resuspend the protein, execute batch-specific calculations and carefully measure the correct amount of solution to add to the media. For cGMP manufacturing, this open operation must be performed in a cleanroom suite under laminar flow. Manufacturing technicians' general sentiment is that such an operation can be disruptive to the manufacturing flow and can cannibalize a significant amount of allowable working time while in the cleanroom. Companies often impose an expiration on gowning of three to four hours to ensure cleanroom technicians pose no impact on the environment and to make sure proper mental and physical rest is granted. Beyond the cost and time considerations, the need to reconstitute and dose powders correctly while using aseptic techniques adds risks. These risks include dosing the material incorrectly, impacting the stability guarantees of the material suppliers, and introducing contamination into the process.
A Closed, Single-Use Solution for Critical Media Ingredients
Akron Bio is focused on supplying cGMP ancillary materials required for cell therapy production in the most practical format possible for the end-user while still ensuring quality and reliability. The company has focused on developing fit-for-purpose products for clinical and commercial manufacturing that minimize the risk of error, decrease processing times, support closed unit operations to mitigate contamination, and potentially move production towards lower grade environments – ultimately reducing manufacturing costs.
Akron Bio was the first company to develop a liquid cGMP Interleukin-2 formulation that is stable at 2-8 °C for up to 2 years, initially packaged in a syringe format to streamline cell therapy manufacturing. Despite the ease-of-use benefits of providing cytokines in liquid format, avoiding the need to reconstitute lyophilized proteins, syringes do not qualify as functionally closed systems and their handling must take place under strict environmental conditions.
At the request of numerous cell therapy developers, Akron Bio focused on developing its plasma-derived products (HSA and virus-inactivated AB), cytokines, and growth factors into a single-use bag container closure system. To achieve this, significant efforts went into developing stable cytokine formulations at dilute concentrations (down to 10 mg/mL) filled to a range of working volumes aligned with end user’s process needs. The liquid formulation technology has been patented by Akron Bio, and the cGMP materials are now part of Akron’s Closed System Solutions(CSS)TM line with several fill volumes and weldable tubing options.
The multiple connection options and purposely chosen fill volumes for both the cGMP-compliant plasma-derived products and liquid cytokines enable the functional closure of unit operations while achieving the primary purpose of COGs reduction for preclinical to commercial ex vivo cell therapy manufacturing platforms. In addition, a Drug Master File with FDA is available for each CSS product, allowing seamless regulatory support.
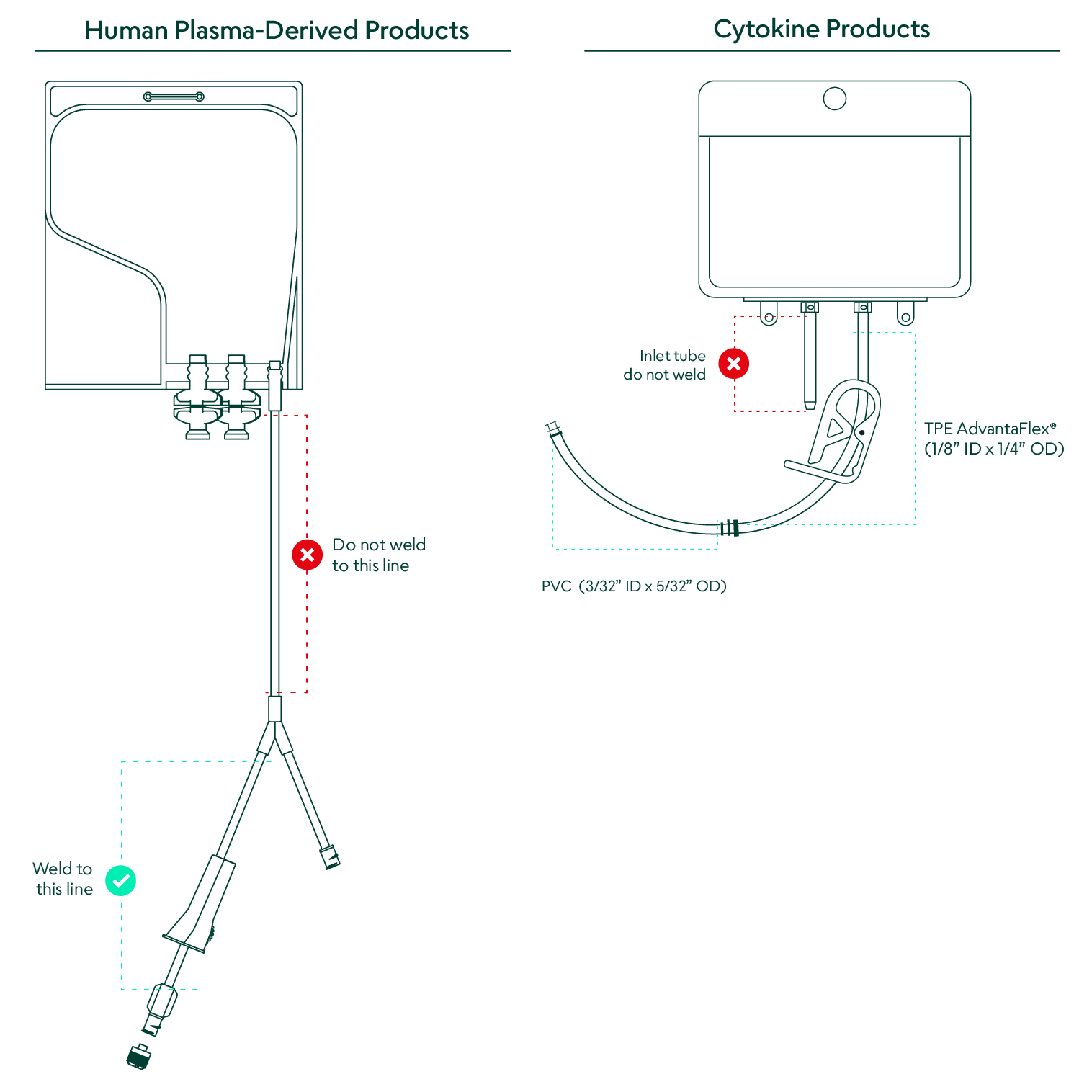
Depending on the specific product, the sterile bag chamber consists of either a fluoropolymer or ethylene-vinyl acetate copolymer, both of which are plasticizer-free, highly inert, provide structural integrity with varying degrees of flexibility, and have been extensively validated, controlled, and qualified. Both the cytokine and plasma-derived products come in bags with weldable polyvinyl chloride (PVC) outlet tubing. The CSS cytokine products also include a TPE weldable tubing option. The human plasma-derived products offer two twist-off spike ports that allow for various attachments and adapters to fit the requirements of each application.
Reduced Risk and Simplified Manufacturing
The availability of cytokine and plasma-derived products in a closed system marks a paradigm shift for cell therapy manufacturing. Access to important raw materials in ready-to-use single-use bags with weldable tubing dramatically reduces manufacturing time and risk due to human error and contamination. Transferring the CSS materials into the process only requires a sterile weld between the outlet tubing on the CSS bag and the receiving process line.
Since sterile welding enables functional closure, these unit operations can potentially be performed in less costly background environments (ISO 7 or 8 vs. ISO 5, for instance) further reducing COGs.
A Broad Product Portfolio
Akron Bio’s Closed System Solutions (CSS)™ cytokines and plasma-derived products can be used in a plug-and-play fashion with existing manufacturing processes. Custom filling configurations and fill volumes can be developed for full batch orders to align to process/delivery requirements.
IL-2 CSS
The amino acid sequence of recombinant Human Interleukin-2 (rHu IL-2) from Akron Bio is comparable to Proleukin® (aldesleukin), and its functional similarity in T cell expansion has been evaluated and confirmed2. It is offered in 1, 2, 7.5, and 15 million international unit aliquots (MIU/bag).
IL-7 and IL-15 CSS (IL-21 Coming Soon)
rHu IL-7 is available in a 100 μg (10 μg/mL) aliquot, while rHu IL-15 is offered in an aliquot size of 50 μg (10 μg/mL). rHu IL-21 is under development and coming soon.
Plasma-Derived CSS
Human Serum Albumin (HSA) 25% from Akron Bio does not contain stabilizers typically found in other pharmaceutical albumin solutions (acetyltryptophanate and caprylate), allowing for an HSA supplement that promotes optimal cell culture performance for the human cell therapy industry. It is available in CSS bags containing 60 or 100 mL.
Akron’s Human AB Serum, Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated (viHABS) is a high-quality, virus-inactivated human AB serum product with great batch-to-batch consistency and a unique safety profile. This plasma-derived product is available in CSS bags containing 60 or 100 mL.
Akron Bio’s mission is to power the future of medicine® and we believe we’re doing so through our robust and innovative products and services. We aim to expand the CSS product portfolio over the coming years to include other core cytokines and growth factors used across multiple cell types in the market. It will take time to develop stable liquid formulations for all these materials, but we at Akron Bio are excited and committed to developing and delivering many more reliable, high-quality, Closed System Solutions (CSS)™ products for a variety of cell types to support forward-thinking cell therapy companies.
References
- Hernandez I, Prasad V, Gellad WF. Total Costs of Chimeric Antigen Receptor T-Cell Immunotherapy. JAMA Oncol. 2018 Jul 1;4(7):994-996. doi: 10.1001/jamaoncol.2018.0977. Erratum in: JAMA Oncol. 2018 Oct 1;4(10):1439. PMID: 29710129; PMCID: PMC6145722.
- J. Cho, J. Ritz, C. Zylberberg, Comparing the functionality of proleukin® and akron interleukin-2 through an analysis of key T cell subsets, Cytotherapy, Volume 22, Issue 5, Supplement, 2020, Pages S121-S122, ISSN 1465-3249, https://doi.org/10.1016/j.jcyt....(https://www.sciencedirect.com/science/article/pii/S1465324920302929)