cGMP ImmunoCell® Growth Medium
ImmunoCell® Medium for the Growth of Human Therapeutic Immune Cells
Akron’s ImmunoCell® Growth Medium (ICGM) product is a serum-free medium formulated for the culture of human T cells intended for therapeutic treatment. Akron developed and launched ICGM as a basal T cell medium with the capacity to promote superior growth in peripheral blood mononuclear cells, isolated monocyte cultures, and T cells. Akron’s ICGM has been successfully evaluated by several partners on their cell therapy platforms using T cells isolated from healthy donors.
ICGM is designed to be serum-independent, and does not require supplementation with serum products. It is also free of cytokines, enabling the user to add the ideal combination and concentration as necessary for their specific cell product. Sterile filtration and aseptic filling are performed as part of the cGMP manufacturing process. Release testing performed to enable the release of each batch includes Mycoplasma, Endotoxin, and Sterility testing on the final product. Our T cell medium is packaged in PETG bottles and available in a 1 L aliquot size. Custom fill in different size bottles and bags are available upon request.
Advantages
- Antibiotic-free
- Serum-free and animal origin component-free
- Manufactured in compliance with cGMP guidelines
- Memory phenotypes of cells are maintained when cultured
- Maintains function when subjected to environmental stresses such as light, elevated temperature, and freeze-thaw cycles
Quality
- Relevant cGMP Guidelines Used in Manufacture, Testing, and Release
- USP <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
- ISO 20399:2022, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products
Stability
- 24-month shelf life
- Store at 2-8 °C
- Transport on cold packs
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
• Appearance (Visual Inspection)
• pH (Potentiometric)
• Osmolality (USP <785>)
• Functional Bioassay (Ex-vivo Jurkat T cell Expansion)
• Mycoplasma (PCR)
• Endotoxin (USP <85> / EP 2.6.14)
• Sterility (USP <71> / EP 2.6.1)
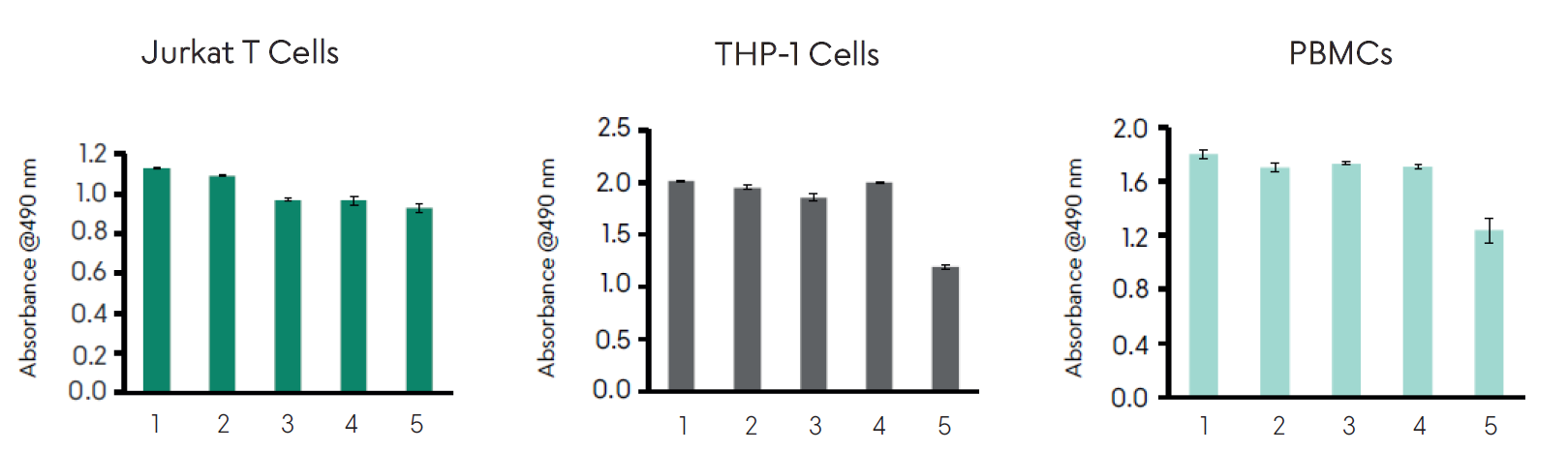
Functional bioassay results for four different lots of Akron’s ICGM (bars 1-4) compared against a commercial competitor T cell medium (bar 5). Jurkat T cells were seeded at 20,000 cells per well and supplemented with IL-2 at 100 units/mL, THP-1 cells were seeded at 30,000 cells per well, and PBMCs were seeded at 300,000 cells per well. Cells were allowed to incubate for 3 days at 37 °C then proliferation was measured using an MTS-based colorimetric viability assay.
1. Why use Akron’s ICGM?
Akron’s ICGM is manufactured following all relevant cGMP guidelines for ancillary materials. ICGM has been shown to promote superior growth in peripheral blood mononuclear cells, isolated monocyte cultures, and T cells. Akron’s ICGM has been successfully evaluated by several partners on their cell therapy platforms using T cells isolated from healthy donors.
2. Do I need to supplement this media?
Akron ICGM is serum free and serum independent, there is no need to supplement with serum products. Moreover, the medium does not contain cytokines to maintain flexibility and ensure utility across a variety of cell types and manufacturing processes. The user is free to supplement as needed to support the intended use.
3. Does Akron provide phenol red free (PRF) ICGM?
Yes, ICGM is available without phenol red upon request.
4. What are the recommended storage conditions?
We recommend storing this product at 2-8 °C.
5. What are the shipping conditions?
This product ships on cold packs.
6. What is the shelf life?
This product is currently under a formal long-term stability program. Informal studies have shown biological functionality is maintained for at least 24 months.
7. Does the product maintain functionality when exposed to extreme conditions?
The product has been shown to maintain biological functionality when heated, frozen, or exposed to light. Heated conditions tested include 45 °C for 1 hour and 20-25 °C for 72 hours, frozen conditions tested were -20 °C for a single freeze thaw cycle, and light exposure testing was performed after 24 hours of exposure.
8. Does this product have a TSE/BSE statement?
Yes, a TSE/BSE statement is available upon request.
9. Are animal-derived materials used in the manufacture of this product?
No animal-derived materials are used in the manufacturing process or in the final product.
10. Are antibiotics used in the formulation or processes in the manufacturing facility?
No antibiotics are included or processed within the manufacturing facility.
11. What safety testing is done on this product?
Each lot of ICGM is tested for Mycoplasma via PCR, Bacterial Endotoxins per USP<85>, and Sterility per USP <71>.
12. Do you have an SDS for this product?
Yes, an SDS is available upon request and is included with every shipment.
13. How does Akron measure functionality for this product?
Each lot of ICGM is tested for biological functionality via an ex vivo Jurkat T cell proliferation bioassay.
14. Which cell types are suitable for culture with Akron’s ICGM?
Akron’s ICGM can be used for the proliferation of a variety of human immune cells. Its effectiveness has been verified in peripheral blood mononuclear cells (PBMCs), isolated monocyte cultures, and T cells.
15. What is the intended use for the product?
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
16. What packaging options are available?
This product is available in a 1 L aliquot size that comes packaged in sterile 1 L PETG bottles. Custom fill sizes in both bottle and bag formats are available upon request.
Aliquot Sizes & Formats
Bottles (1000 mL) Cat # AK9985-1000