cGMP rHu IL-7 CSS
rHu IL-7 outfitted for closed-system manufacturing.
See why everyone is choosing Akron cytokines.
Akron’s Recombinant Human Interleukin-7 (rHu IL-7) Closed System Solutions (CSS)™ is manufactured following all relevant cGMP guidelines for ancillary materials. The rHu IL-7 active substance is supported by a Type II Master File (MF) on file with the FDA, which can be referenced during your drug or biologic application process.
Akron’s rHu IL-7 is a single chain, 17.5 kDa, non-glycosylated cytokine analog expressed in E. coli, containing 153 amino acids. The downstream purification process uses a multi-step orthogonal approach, without the use of affinity tags, to minimize exogenous impurities and ensure the delivery of highly purified and active substance for further manufacturing applications.
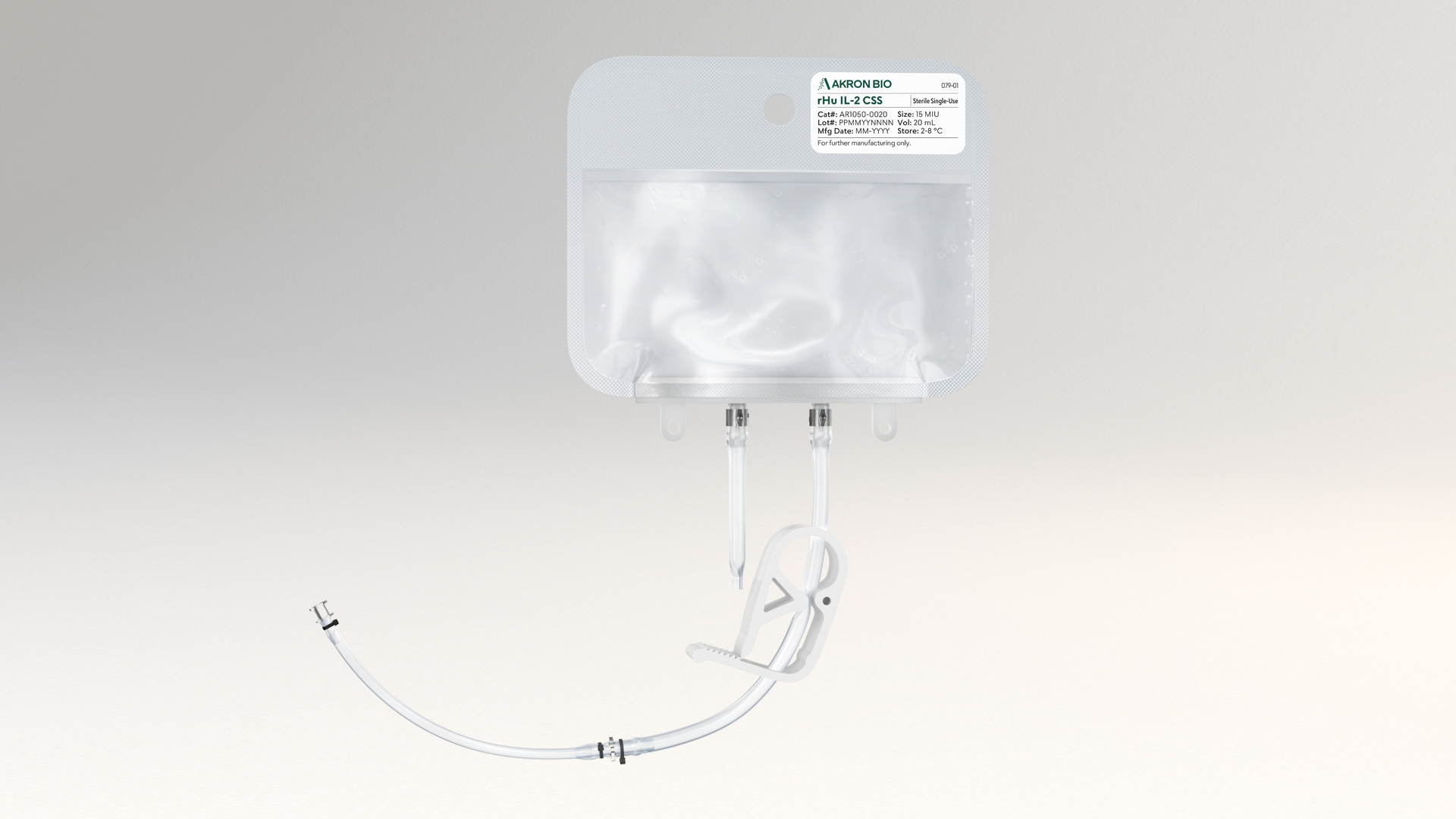
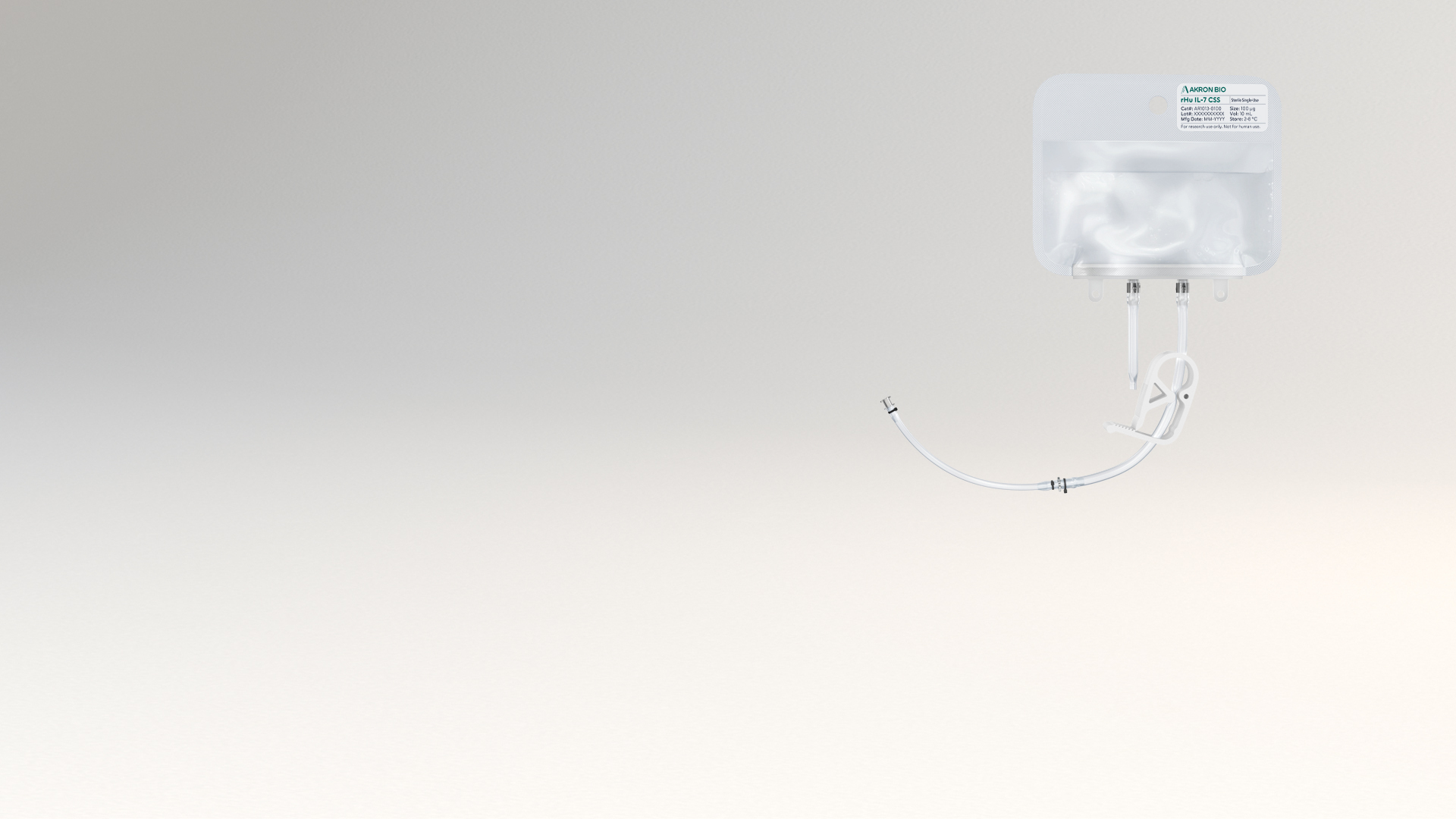
The liquid rHu IL-7 CSS product is packaged in a sterile fluoropolymer bag with two different weldable tubing connection options, allowing for easy incorporation into modern closed-system cell culture bioprocessing protocols. rHu IL-7 CSS increases safety and ease of use by eliminating the reconstitution step during manufacture and allows for the introduction of cytokine material into culture media in a fully contained manner to maximize sterility. Sterile filtration and aseptic filling are followed by Endotoxin and Sterility testing performed per USP/EP on the final product. See product Packaging features below.
Active Substance
- Type II eCTD MF (#026084) on file with FDA
- Carrier protein-free formulation
- All raw materials are compliant, controlled, and traceable under Akron’s Quality Management System (QMS)
Manufacturing
- Multi-step downstream purification strategy excluding affinity tags
- Commercial-scale production capacity
- E. coli expression system
- Sterile filtration and aseptic filling
Packaging
- Sterile bag chamber – 8-mil fluoropolymer film providing inert bio-reactivity, structural integrity, and minimal gas transmission
- Two-part tube weldable tube - proximal 6” portion made from weldable TPE AdvantaFlex® (1/8” ID x 1/4” OD), and the distal 6” portion made from standard weldable PVC (3/32” ID x 5/32” OD)
- Primary packaging is plasticizer free
- Primary packaging materials extensively validated, controlled, and qualified to ensure a consistent experience
- Every bag packed in individual protective cassette and shipped in validated CSafe Parcel insulated shipper
Quality
- Relevant cGMP guidelines used in manufacture, testing, and release
- USP <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
- EP 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products
- ISO 20399:2022, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products
Stability
- Under long-term stability program
- Store at 2-8 °C
- Transport with cold packs
- Do not freeze
Methods of Use
There are two different options available for connecting to and extracting the liquid solution from Akron’s rHu IL-7 CSS product (for full instructions, see “Methods of Use” document):
1) Weldable connection via proximal TPE AdvantaFlex® section (1/8” ID x 1/4” OD)
2) Weldable connection via distal PVC section (3/32” ID x 5/32” OD)
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications only. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
NOTICE: Catalog # AR1013 is patent pending.
• Appearance
• pH
• ID via SDS-PAGE
• Biological Activity
• Purity via Reducing SDS-PAGE
• Purity via Non-reducing SDS-PAGE
• Bacterial Endotoxins
• Sterility
IL-7 is primarily expressed by non-marrow-derived stromal and epithelial cells and is required for early T cell development inside the thymus and for T cell homeostasis and regeneration in the periphery. It promotes the differentiation of hematopoietic stem cells (HSCs) down the lymphoid cell lineage and optimizes T celldendritic cell interaction. The dimeric IL-7 receptor protein (IL-7R) shares an identical subunit with the IL-2, IL-15, and IL-21 receptor proteins and activates some of the same signal transduction mechanisms. IL-7 is currently being investigated as an immunotherapy agent for various malignancies as well as HIV infection. Akron’s cGMP-compliant rHu IL-7 can be used to stimulate proliferation of cytotoxic T lymphocytes (CTLs), B lymphocyte cells, monocytes, NK cells, and LAK cells.
Aliquot Sizes & Formats
Bags (100 μg) Cat. # AR1013-0100 (10 μg/mL)