Critical Raw Materials Elevated
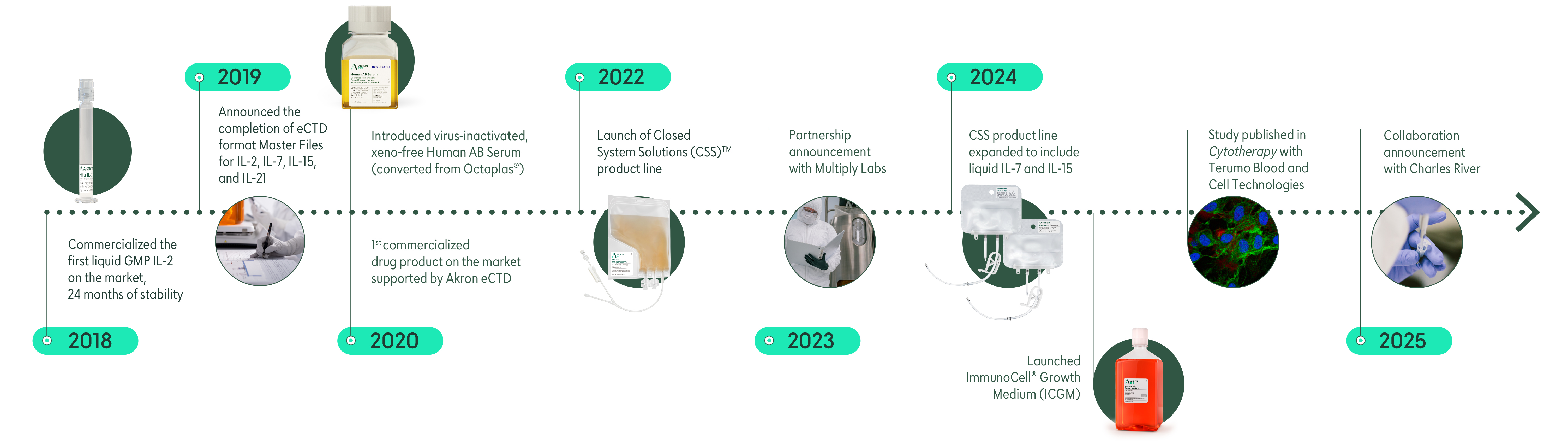
Since 2006, we’ve been a leading manufacturer of critical raw materials, providing hundreds of cutting-edge cell and gene therapy companies with the materials they need to develop tomorrow’s cures.
Our success and reputation as a leading provider in the space is due to a deep understanding that the supply of high-quality, well-documented raw materials is critical to the scale-up and commercialization of lifesaving therapies.
Recent Innovations at Akron
Revolutionizing Critical Raw Materials
As a key critical raw materials supplier and manufacturer we’ve constantly pushed the raw materials space into the future to ensure our clients have the highest-quality building blocks to develop and commercialize future therapies.
Closed System Solutions (CSS)™
Our shelf-stable liquid cytokines and human plasma-derived blood products are available in single-use, ready-to-use bags with weldable tubing to reduce human error by closing manufacturing processes.
Enhanced Regulatory Support
Our keystone raw materials are backed by eCTD master files and all of our products include our enhanced regulatory support giving your organization a critical edge on the path to commercialization.
Multiply Labs & Akron Partner
In December 2023, we announced a partnership with Multiply Labs, a robotic company to automate the use of cGMP-compliant liquid cytokines in cell therapy with the goal of simplifying delivery and reducing risks to the patient.