The importance of cytokine and growth factor purity in cell therapy development and manufacturing
Today, it is more important than ever to understand the quality of ancillary materials in the cell and gene therapy supply chain. As part of a therapy developer’s risk and impact assessment, each material must be evaluated for purity, lot-to-lot consistency, and potential impact on the drug product. Because of its outsized impact on cost of goods, the quality of cytokines and growth factors is critical to a therapy’s ultimate success. This blog focuses on the importance of cytokine and growth factor purity.
There are several purification methods employed in recombinant protein development and production, including affinity chromatography and ion exchange chromatography, among others. The method employed is secondary to overall strategy, as far as protein purity is concerned. Nevertheless, certain methods are associated with strategies that confer higher-quality proteins than others.
Because cytokine and growth factor manufacturing companies have traditionally focused on the research market, many of them utilize affinity chromatography. This purification method is excellent for protein capture, but may or may not be followed by additional purification steps to attain higher degrees of purity. Furthermore, when the histidine tag is cleaved from the nickel column, nickel residue can remain in the product (this process is not permitted for injectable-grade cytokines). Affinity chromatography tends to be less costly, given the number of purification steps typically involved.
At Akron, we develop and manufacture our cGMP-compliant cytokines without the use of affinity chromatography. We take an orthogonal approach to protein purification, a strategy that includes protein capture, intermediate purification, and polishing steps:
- The capture stage includes the isolation, concentration, and stabilization of the target protein in a way that preserves its potency.
- The intermediate purification stage focuses on the removal of other proteins and nucleic acids, endotoxins, and viruses.
- In the polishing stage, remaining trace impurities are removed, and the target protein may be transferred to conditions suitable for use or storage. The objective is to achieve final purity.
At Akron, each of these stages may include the use of several columns, generating a relatively lengthy process that ultimately produces high-quality proteins. Because of our ability to manufacture and purify our cytokines and growth factors with pharmaceutical processes, the proteins we manufacture are often the cleanest on the market. See below for SDS-PAGEs generated in-house, comparing our suite of recombinant human interleukin-7, 15, and 21 to other GMP products on the market.
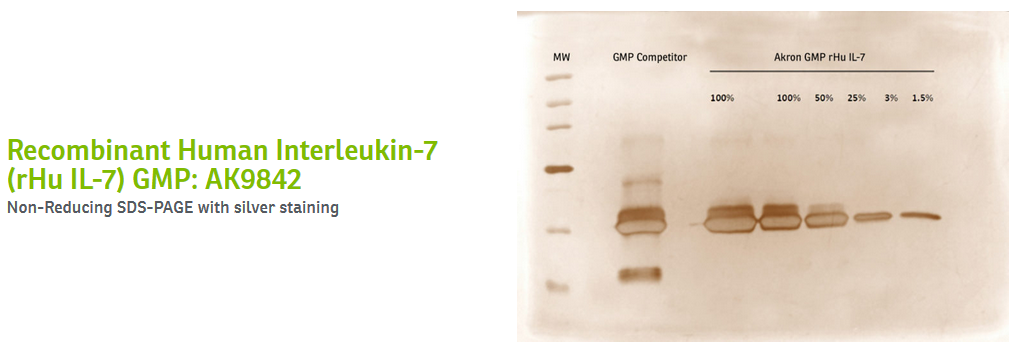

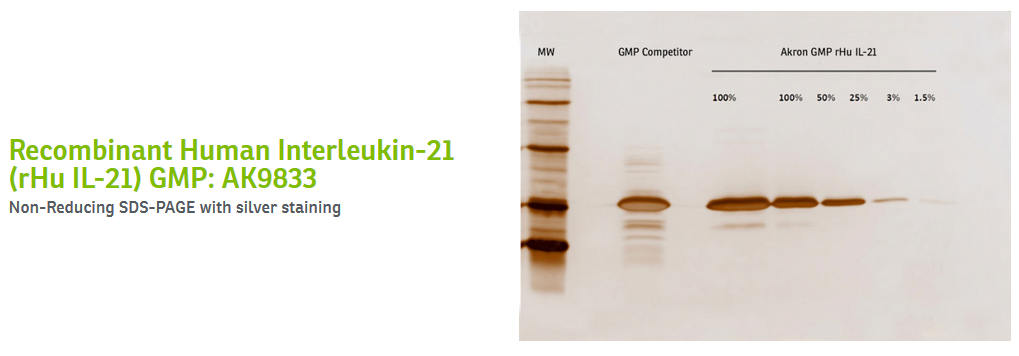
Certain strategies can be more conducive to the development of a highly pure protein than others. Affinity chromatography, in and of itself, can be a perfectly useful purification method. Indeed, it is highly efficient and cost-effective in generating large amounts of protein. However, without subsequent steps to cleave the tag and ensure the removal of impurities, the final product may lack the quality that cell and gene therapy developers should seek for their manufacturing processes, especially as they move closer to commercialization.
We are hopeful that FDA, EMA, PMDA, and other regulatory authorities will eventually raise the bar, ensuring that all cytokines utilized in the advanced therapy development and production process are purified using techniques and strategies that support greater therapeutic integrity. At Akron, we leverage our pharmaceutical manufacturing know-how and infrastructure to generate the highest-quality cytokines and growth factors on the market. You can take our word for it or try them out for yourself. Please reach out for more information, or to request samples.