Superiority, Non-inferiority and Equivalence – The Phase III Question
Clinical trials occur in phases – Phase I through Phase III – and if successful, culminate in FDA approval to market a drug product. In the US, Phase III trials generally assess if a new treatment method or drug product is as effective or better than existing treatments or products. In short, Phase III trials are devised to determine superiority or non-inferiority or equivalence. The objective of a Phase III trial must be defined up front; is the end goal to determine superiority or non-inferiority or equivalence? Here, we unpack what each of these mean.
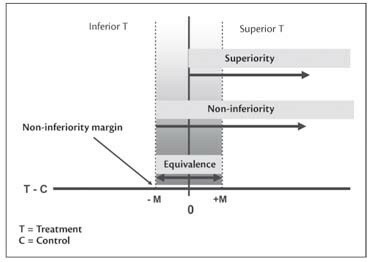
The aim of demonstrating superiority implies showing that the treatment or product is ‘superior’ to another treatment, which can be the current standard treatment (called an active control) or even a placebo. The data from these trials are assessed for statistical significance That is, do the data show a difference between the treatments or products. While a treatment or product being evaluated in a Phase III trial can be shown to be superior to another if results are statistically significant, this does not necessarily mean that the findings have clinical relevance. Statistical significance is a mathematical confirmation that the sample size is adequate to sufficiently determine if the data shows that a visible effect exists within the sample. Superiority is shown statistically when the difference between the mean of the new treatment and the mean of the standard treatment is not equal to zero within a 95% two sided confidence interval. The difference between their means are different statistically to a significance of 5% (p=0.05).
Non-inferiority trials aim to show that the treatment is not ‘inferior’ or worse in efficacy to another treatment, which can be the current standard treatment. In these trials, the treatment under development may not be more efficacious than the current standard treatment, but may offer improved safety or convenience, or may bring down costs, making the treatment more affordable. An important aspect of a Phase III non-inferiority trial is establishing that the compared treatment has a defined effect at a defined sample size. This is to ensure that the assessed treatment or product is not being compared to a treatment that is also inferior; this is to avoid approving an ineffective study. The statistical approach to data in non-inferiority trials is a one-sided confidence interval approach. The difference between the mean of the new treatment and the mean of the standard treatment should be greater than the lower equivalence margin.
The objective of equivalence trials is to show that the two treatments being compared differ by a value that is clinically unimportant. The difference between the mean of the new treatment and the mean of the standard treatment should be within the upper and lower equivalence margin of the 95% two sided confidence intervals.
ICH E9 and E10 discusses the requirements for clinical trials with regards to proving superiority, non-inferiority or equivalence required for FDA approval of a treatment method or product. Please review these documents to learn more, and check out our website to see how our team’s regulatory support enables the clinical development of advanced therapies.