Stoichiometry via mass spectrometry sheds light on the T cell metabolism
The field of immunometabolism has attracted growing attention in recent years due to the high potential for the development of cancer immunotherapies. Upon activation, T lymphocytes (T cells) undergo significant metabolic changes that allow them to mediate immune responses. Stimulating these and other immune cells has important outcomes beyond energy production (Fig. 1).
A key regulator of T cell differentiation is the rapamycin complex 1 (mTORC1), a nutrient sensor which controls protein synthesis and turnover, thus affecting T cell subsets differently. For example, mTORC1 inhibition restrains the first cell cycle entry of immune-activated naïve T cells but has little impact on the proliferation of rapidly cycling effector T cells.
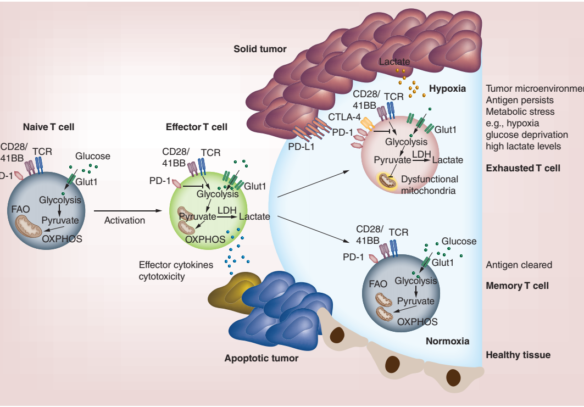
Figure 1. T cell activation and metabolism. Left hand side: Glucose transporter-1 (Glut-1) is upregulated and glycolysis is increased in effector cells upon activation allowing T cells to rapidly divide and produce effector cytokines and cytotoxic mediators. Lower right-hand side: Antigens clear and Memory T cells revert to a metabolism similar of that of naïve cells, but remodel their mitochondria allowing them to rapidly produce energy for a recall response upon antigen re-encounter. Upper right-hand side: Despite upregulating Glut-1, exhausted T cells cannot take up enough energy and show mitochondrial dysfunction, express high levels of co-inhibitory receptors, and show low capacity for proliferation and effector function.
Analyzing the entire set of proteins expressed by T cells is the only way to understand the full effect of immune activation on T cells. In this study, scientists from the University of Dundee utilized quantitative mass spectrometry to reveal how CD4+ and CD8+ T cells restructure proteomes in response to antigen and mammalian targets of mTORC1. T cell activation not only increased transcriptional reprogramming but also global proteome remodeling. These data reveal the reason for divergent outcomes of mTORC1 inhibition on cell cycle progression in naïve versus effector T cells and demonstrate how immune activation clearly shapes effector T cell fate. This same mechanistic approach should be able to shed a light on how CAR-T response to the immune suppressive tumor microenvironment is related to hypoxia and resulting metabolic changes.
Echoing the 2019 Noble Prize awarded for hypoxia research, this research provides a new avenue for future therapeutic approaches such as specific pharmacological inhibitors that transform T cells and thereby increase tumor response to immune checkpoint inhibitors that keep immune responses in check. It has great potential to address the challenging solid tumor microenvironment or the systemic challenge of autoimmune disease. To read more, please see the full paper, available via this link.