Exosomes as promising vehicles for drug delivery to the brain
The blood brain barrier (BBB) is an important physiological wall protecting the central nervous system (CNS) from potentially harmful materials in the blood. However, this protective mechanism also blocks the entry of many drugs. The brain is one of the most challenging organs to access, often requiring the use of fine-tuned and precise drug delivery systems.
There are three types of barriers within the BBB. The first is physical – the tight junction that blocks ions and other hydrophilic substances. The second is the transport barrier – a membrane efflux pump such as P-glycoprotein that removes metabolic waste and other exotic substances. The third is the enzymatic barrier, which is comprised of the enzymes in the brain that metabolize toxic materials. Over 98% of small molecule drugs and 100% of proteins, peptides and RNA/DNA cannot penetrate the BBB. Even recently developed nanoparticles have had limited success in delivering drugs to the brain. There is an urgent need to develop novel therapeutic tools to improve drug delivery to the brain.
Biocompatibility, biodegradability and low immunogenicity are basic requirements of brain drug delivery systems. Natural nanostructure molecules known as exosomes are promising candidates. There is a great deal of scientific evidence that exosomes can cross or bypass the BBB with or without surface modification both in vivo and in vitro. Exosomes are defined as small vesicles ranging from 30-100nm in size that are found in nearly all eukaryotic fluids and facilitate a range of important cellular functions.
The recently accepted review entitled “Harnessing Exosomes for the Development of Brain Drug Delivery Systems” describes the current state of scientific knowledge regarding how exosomes cross the BBB, identifies how they package molecular entities of different kinds, and lays out the challenges and opportunities that lie ahead. There are still many puzzles to be solved. For starters, we don’t have a full understanding of how exosomes achieve intercellular communication within the brain. Furthermore, we don’t know how peripheral exosomes access the brain and deliver their cargo into the CNS. The clarification of the above mechanisms would have an important impact on exosome-mediated drug delivery to the brain. In addition, the drug loading approaches, source selection, stability, and targeting properties of exosomes are major factors that determine their value as brain drug delivery systems.
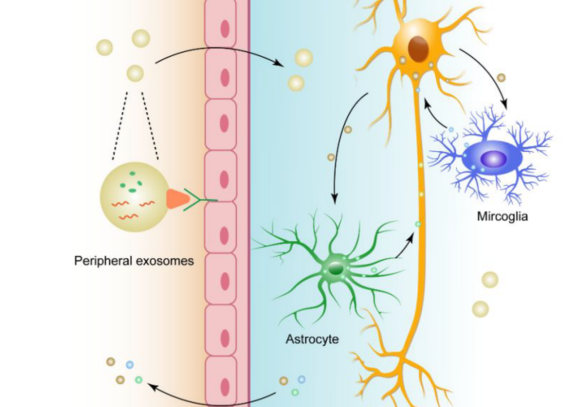
Exosomes can cross the BBB in several ways. Natural exosomes can adhere to different cells (because of their lipidic composition), in this case being taken up by neuronal cells (See Figure above). Another way of crossing the BBB is through exosome surface modification. Here, scientists transfect the progenitor cells with the fusion construct of brain targeted peptide-encoded gene and the exosomal marker gene to generate exosomes with the ability to cross the BBB. These modified exosomes can deliver siRNA in the brain and have shown clear knock-down efficiency at targeting neurons, microglia, and oligodendrocytes. Lastly, one can deliver exosomes via intranasal administration, which is a noninvasive way to deliver exosomes into the brain. Exosomes from human bone marrow-derived Mesenchymal stem cells can reach hippocampus in mice 6 hours after intranasal administration.
Beyond discussing ways to cross the BBB, the review paper outlines various strategies for loading drugs into exosomes. A critical factor to consider for drug delivery is stability and clearance in the body. Normally, most exosomes are cleared within 6 hours of intravenous injection. It has been reported that the use of polyethylene glycol (PEGylation) on exosome surface can extend their time in circulation, and therefore efficacy. Several experiments use CD47 as a ligand, which releases a “don’t eat me” signal to prevent exosome-phagocytic cell clearance and therefore increase efficacy over time.
Finally, large amounts of high-quality exosomes are needed for the purpose of developing clinical drug delivery systems. Manufacturing exosomes at industrial scale is challenging. New microcarrier-based 3D cell culture and tangential flow filtration combined with good manufacturing practices (GMPs) enable the production of high-quality exosomes at scale.
In summary, although the exosome seems to be a promising vehicle for drug delivery to the brain, there is still work to be done in order to manufacture these carriers at scale. For many patients who suffer from brain related diseases such as Parkinson’s, Alzheimer’s and others, there is hope in the near future. These novel delivery systems will be more efficient at crossing the BBB, leading to improved treatment options.