Advancements in biomedical engineering: developing hydrogels for tissue regeneration and drug delivery
Hydrogels are a 3-D network of highly absorbent polymers that have been investigated since the late 20th century for various biomedical applications, including drug delivery, wound healing, and tissue engineering. Their highly absorbent nature gives them a flexibility similar to natural tissues and allows oxygen, nutrients, proteins, and other biomolecules to readily enter and nourish the cells within. Hydrogels vary on the basis of their source material and biodegradability. Specifically, natural hydrogels more closely mimic the extracellular matrix while synthetic polymers are tunable to enable specific cell-matrix interactions. Further, certain types of therapies may require a hydrogel that is non-biodegradable, such as contact lenses, while others require hydrogels that biodegrade, such as joint cartilage 3-D scaffold hydrogels.
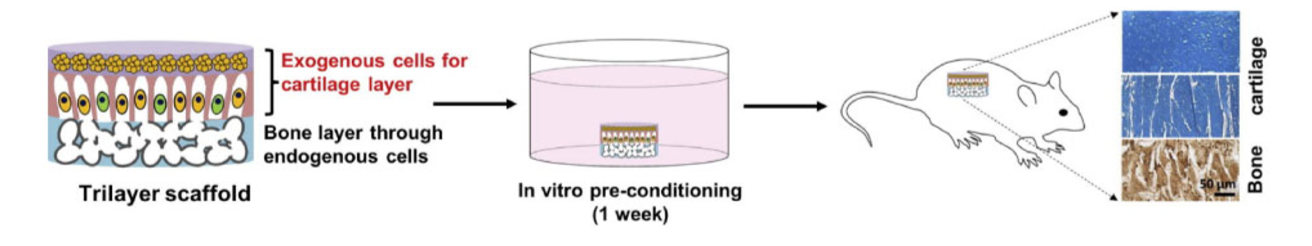
Cartilage regeneration remains a challenge in tissue engineering due to its avascular, aneural, and non-lymphatic characteristics that limit even self-regeneration and intrinsic repair. Additionally, with four different zones of tissue with different cell morphologies, compositions, structures and mechanical properties, damaged cartilage, especially in the joints, is difficult to repair artificially. Due to the multiple zones of tissue, research has shifted from focusing on single layer to multilayer and interpenetrating polymer network (IPN) hydrogels (Figure 1). IPNs have shown enhanced mechanical strength when compared to single layer hydrogels, and certain studies also investigated adding nanoparticles to hydrogel scaffolds in order to improve their stiffness, thereby making them more like natural cartilage. (Li et al., 2019)
Furthermore, protein-based hydrogels derived from natural tissues—including plasma, skin appendages, and cells grown in vitro—are used extensively in medicine, including in cartilage regeneration, because they mimic the natural extracellular matrices of mammalian tissue. However, they present challenges because, while they are able to form structures ranging from nanoscales all the way up to macroscales, their native structure greatly determines their function. Further identifying the secondary, tertiary, and quaternary structures of protein-based biopolymers holds great potential to lead to a wide-spread use of protein-based hydrogels derived from natural tissues. (Jabbari, 2019)
Drug delivery is another obstacle that hydrogels are being developed to overcome. Drug delivery is usually conducted orally, but some medications face lowered bioavailability due to pH variations in the GI tract and their being partially metabolized by the liver. While delivering drugs via the skin presents challenges, Ngo et al. (2019) developed a film-forming hydrogel (FFH) that is flexible, wear-resistant, and allows prolonged drug release of even marginally water-soluble drugs. They studied drug delivery of curcumin (Cur), a polyphenol that targets signaling molecules associated with conditions including arthritis and eye degeneration. The researchers loaded the Cur into gold nanoparticles (CUR-GNPs) in order to decrease the size of the particles and enable easier diffusion across the skin barrier. Decreasing the size of the drug particles loaded into the FFH increased drug permeation from 12% to 85% compared to pure drug in the FFH alone. Hydrogels enable new approaches to drug delivery, wound healing, and tissue engineering.