Akron's Closed System Solutions™ (CSS) Line of cGMP Products
Say goodbye to wasted time and human error with ready-to-use cytokines and media supplements in single-use systems
Our CSS cGMP BioProducts are available in single-use, ready-to-use bags with weldable tubing, allowing plug-and-play integration into modern closed-system manufacturing protocols. The current CSS offerings leverage existing cGMP manufacturing processes and have Drug Master Files with the FDA for seamless regulatory support. Available now in IL-2, IL-7, HSA, and viAB with various fill volumes and concentrations, enabling preclinical to commercial manufacturing.
Benefits of using Akron’s Closed System Solutions™ products

Test our CSS BioProducts today
IL-2, HSA, and viAB are now available to ship. Contact us today to request a sample.
FAQ's
Akron’s novel CSS liquid formulation and sterile bag packaging increase safety and ease of use by eliminating the reconstitution step during manufacture, allowing for the direct introduction of cytokine material into culture media in a fully contained manner.
Yes, the bags include tubing that can be used to make weldable connections to your closed-system process line (for full instructions, see the “Methods of Use” document on the product pages).
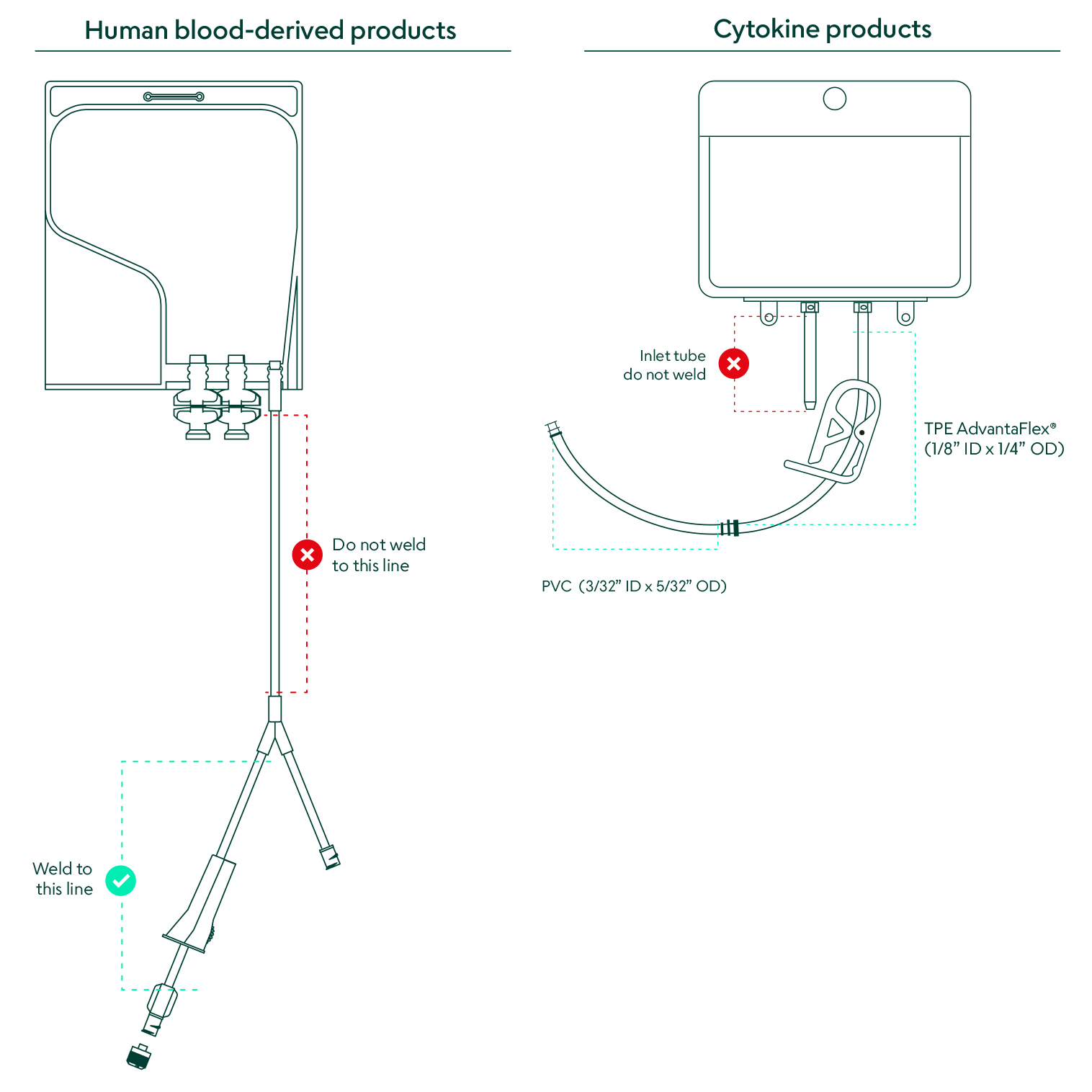
The cytokines bag includes both TPE AdvantaFlex® tubing and PVC tubing. The human blood-derived products include only PVC tubing.
Akron's CSS cytokines are packaged in a sterile 25 mL fluoropolymer bag chamber. The outlet tube is made from two different weldable materials to choose from. The top portion is made from TPE AdvantaFlex® (1/8” ID x 1/4” OD) and the bottom portion is made from standard weldable PVC (3/32” ID x 5/32” OD). Akron's CSS human blood-derived products comes packaged in a sterile bag chamber made from Ethylene-vinyl acetate (EVA) for inert bioreactivity and increased flexibility. The 6” outlet tubing on the distal end of the Y connector is made from weldable polyvinyl chloride (PVC) (3.0 mm ID x 4.1 mm OD). These packaging materials are extensively validated, controlled, and qualified to ensure a consistent experience.
We do not recommend freezing this product as there is PVC tubing on this product.